This is a preprint.
Allosteric Neutralization by Human H7N9 Antibodies
- PMID: 37986867
- PMCID: PMC10659534
- DOI: 10.21203/rs.3.rs-3429355/v1
Allosteric Neutralization by Human H7N9 Antibodies
Update in
-
Human neutralizing antibodies target a conserved lateral patch on H7N9 hemagglutinin head.Nat Commun. 2024 May 27;15(1):4505. doi: 10.1038/s41467-024-48758-4. Nat Commun. 2024. PMID: 38802413 Free PMC article.
Abstract
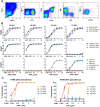
The avian influenza A virus H7N9 causes severe human infections with more than 30% fatality despite the use of neuraminidase inhibitors. Currently there is no H7N9-specific prevention or treatment for humans. From a 2013 H7N9 convalescent case occurred in Hong Kong, we isolated four H7 hemagglutinin (HA)-reactive monoclonal antibodies (mAbs) by single B cell cloning, with three mAbs directed to the HA globular head domain (HA1) and one to the HA stem region (HA2). Two clonally related HA1-directed mAbs, H7.HK1 and H7.HK2, potently neutralized H7N9 and protected mice from a lethal H7N9/AH1 challenge. Cryo-EM structures revealed that H7.HK1 and H7.HK2 bind to a β14-centered surface partially overlapping with the antigenic site D of HA1 and disrupt the 220-loop that makes hydrophobic contacts with sialic acid on the adjacent protomer, thus affectively blocking viral entry. The more potent mAb H7.HK2 retained full HA1 binding and neutralization capacity to later H7N9 isolates from 2016-2017, which is consistent with structural data showing that the antigenic mutations of 2016-2017 from the 2013 H7N9 only occurred at the periphery of the mAb epitope. The HA2-directed mAb H7.HK4 lacked neutralizing activity but protected mice from the lethal H7N9/AH1 challenge when engineered to mouse IgG2a enabling Fc effector function in mice. Used in combination with H7.HK2 at a suboptimal dose, H7.HK4 augmented mouse protection. Our data demonstrated an allosteric mechanism of mAb neutralization and augmented protection against H7N9 when a HA1-directed neutralizing mAb and a HA2-directed non-neutralizing mAb were combined.
Conflict of interest statement
Competing interests An U.S. provisional patent titled “Human Protective Neutralizing and Non-neutralizing Antibodies and Their Use against Influenza Viruses” was filed with filing No. 63/578,505 and XW, MJ, NCM, HL, DDH, KY, KKT, PDK, and LS as co-inventors.
Figures

References
-
- Gao R., Cao B., Hu Y., Feng Z., Wang D., Hu W., Chen J., Jie Z., Qiu H., Xu K., Xu X., Lu H., Zhu W., Gao Z., Xiang N., Shen Y., He Z., Gu Y., Zhang Z., Yang Y., Zhao X., Zhou L., Li X., Zou S., Zhang Y., Li X., Yang L., Guo J., Dong J., Li Q., Dong L., Zhu Y., Bai T., Wang S., Hao P., Yang W., Zhang Y., Han J., Yu H., Li D., Gao G. F., Wu G., Wang Y., Yuan Z., Shu Y., Human infection with a novel avian-origin influenza A (H7N9) virus. N Engl J Med 368, 1888–1897 (2013). - PubMed
-
- Bao C. J., Cui L. B., Zhou M. H., Hong L., Gao G. F., Wang H., Live-animal markets and influenza A (H7N9) virus infection. N Engl J Med 368, 2337–2339 (2013). - PubMed
-
- Chen Y., Liang W., Yang S., Wu N., Gao H., Sheng J., Yao H., Wo J., Fang Q., Cui D., Li Y., Yao X., Zhang Y., Wu H., Zheng S., Diao H., Xia S., Chan K. H., Tsoi H. W., Teng J. L., Song W., Wang P., Lau S. Y., Zheng M., Chan J. F., To K. K., Chen H., Li L., Yuen K. Y., Human infections with the emerging avian influenza A H7N9 virus from wet market poultry: clinical analysis and characterisation of viral genome. Lancet 381, 1916–1925 (2013). - PMC - PubMed
-
- Su S., Gu M., Liu D., Cui J., Gao G. F., Zhou J., Liu X., Epidemiology, Evolution, and Pathogenesis of H7N9 Influenza Viruses in Five Epidemic Waves since 2013 in China. Trends Microbiol 25, 713–728 (2017). - PubMed
-
- Wang X., Jiang H., Wu P., Uyeki T. M., Feng L., Lai S., Wang L., Huo X., Xu K., Chen E., Wang X., He J., Kang M., Zhang R., Zhang J., Wu J., Hu S., Zhang H., Liu X., Fu W., Ou J., Wu S., Qin Y., Zhang Z., Shi Y., Zhang J., Artois J., Fang V. J., Zhu H., Guan Y., Gilbert M., Horby P. W., Leung G. M., Gao G. F., Cowling B. J., Yu H., Epidemiology of avian influenza A H7N9 virus in human beings across five epidemics in mainland China, 2013–17: an epidemiological study of laboratory-confirmed case series. The Lancet. Infectious diseases 17, 822–832 (2017). - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

