This is a preprint.
A spatial human thymus cell atlas mapped to a continuous tissue axis
- PMID: 37986877
- PMCID: PMC10659407
- DOI: 10.1101/2023.10.25.562925
A spatial human thymus cell atlas mapped to a continuous tissue axis
Update in
-
A spatial human thymus cell atlas mapped to a continuous tissue axis.Nature. 2024 Nov;635(8039):708-718. doi: 10.1038/s41586-024-07944-6. Epub 2024 Nov 20. Nature. 2024. PMID: 39567784 Free PMC article.
Abstract
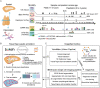
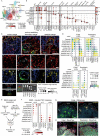
T cells develop from circulating precursors, which enter the thymus and migrate throughout specialised sub-compartments to support maturation and selection. This process starts already in early fetal development and is highly active until the involution of the thymus in adolescence. To map the micro-anatomical underpinnings of this process in pre- vs. post-natal states, we undertook a spatially resolved analysis and established a new quantitative morphological framework for the thymus, the Cortico-Medullary Axis. Using this axis in conjunction with the curation of a multimodal single-cell, spatial transcriptomics and high-resolution multiplex imaging atlas, we show that canonical thymocyte trajectories and thymic epithelial cells are highly organised and fully established by post-conception week 12, pinpoint TEC progenitor states, find that TEC subsets and peripheral tissue genes are associated with Hassall's Corpuscles and uncover divergence in the pace and drivers of medullary entry between CD4 vs. CD8 T cell lineages. These findings are complemented with a holistic toolkit for spatial analysis and annotation, providing a basis for a detailed understanding of T lymphocyte development.
Conflict of interest statement
Conflict of interest J.C.M has been an employee of Genentech, Inc. since September 2022. In the past three years, S.A.T. has consulted for or been a member of scientific advisory boards at Qiagen, Sanofi, GlaxoSmithKline, and ForeSite Labs. She is a consultant and equity holder for TransitionBio and EnsoCell. The remaining authors declare no competing interests.
Figures




References
-
- Pellicci D. G., Koay H.-F. & Berzins S. P. Thymic development of unconventional T cells: how NKT cells, MAIT cells and γδ T cells emerge. Nat. Rev. Immunol. 20, 756–770 (2020). - PubMed
-
- Lobach D. F. & Haynes B. F. Ontogeny of the human thymus during fetal development. J. Clin. Immunol. 7, 81–97 (1987). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
