This is a preprint.
The HIV-1 capsid serves as a nanoscale reaction vessel for reverse transcription
- PMID: 37986899
- PMCID: PMC10659366
- DOI: 10.1101/2023.11.08.566350
The HIV-1 capsid serves as a nanoscale reaction vessel for reverse transcription
Update in
-
The HIV-1 capsid serves as a nanoscale reaction vessel for reverse transcription.PLoS Pathog. 2024 Sep 3;20(9):e1011810. doi: 10.1371/journal.ppat.1011810. eCollection 2024 Sep. PLoS Pathog. 2024. PMID: 39226318 Free PMC article.
Abstract
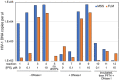
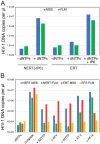
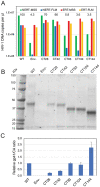
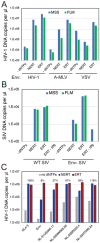
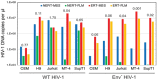
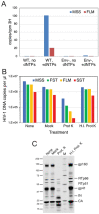
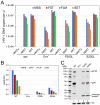
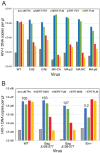
The viral capsid performs critical functions during HIV-1 infection and is a validated target for antiviral therapy. Previous studies have established that the proper structure and stability of the capsid are required for efficient HIV-1 reverse transcription in target cells. Moreover, it has recently been demonstrated that permeabilized virions and purified HIV-1 cores undergo efficient reverse transcription in vitro when the capsid is stabilized by addition of the host cell metabolite inositol hexakisphosphate (IP6). However, the molecular mechanism by which the capsid promotes reverse transcription is undefined. Here we show that wild type HIV-1 particles can undergo efficient reverse transcription in vitro in the absence of a membrane-permeabilizing agent. This activity, originally termed "natural endogenous reverse transcription" (NERT), depends on expression of the viral envelope glycoprotein during virus assembly and its incorporation into virions. Truncation of the gp41 cytoplasmic tail markedly reduced NERT activity, indicating that gp41 permits the entry of nucleotides into virions. Protease treatment of virions markedly reduced NERT suggesting the presence of a proteinaceous membrane channel. By contrast to reverse transcription in permeabilized virions, NERT required neither the addition of IP6 nor a mature capsid, indicating that an intact viral membrane can substitute for the function of the viral capsid during reverse transcription in vitro. Collectively, these results demonstrate that the viral capsid functions as a nanoscale container for reverse transcription during HIV-1 infection.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
