This is a preprint.
Functional parcellation of the neonatal brain
- PMID: 37986902
- PMCID: PMC10659431
- DOI: 10.1101/2023.11.10.566629
Functional parcellation of the neonatal brain
Update in
-
Functional parcellation of the neonatal cortical surface.Cereb Cortex. 2024 Jan 31;34(2):bhae047. doi: 10.1093/cercor/bhae047. Cereb Cortex. 2024. PMID: 38372292 Free PMC article.
Abstract
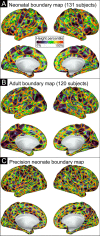
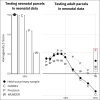
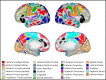
The cerebral cortex is organized into distinct but interconnected cortical areas, which can be defined by abrupt differences in patterns of resting state functional connectivity (FC) across the cortical surface. Such parcellations of the cortex have been derived in adults and older infants, but there is no widely used surface parcellation available for the neonatal brain. Here, we first demonstrate that adult- and older infant-derived parcels are a poor fit with neonatal data, emphasizing the need for neonatal-specific parcels. We next derive a set of 283 cortical surface parcels from a sample of n=261 neonates. These parcels have highly homogenous FC patterns and are validated using three external neonatal datasets. The Infomap algorithm is used to assign functional network identities to each parcel, and derived networks are consistent with prior work in neonates. The proposed parcellation may represent neonatal cortical areas and provides a powerful tool for neonatal neuroimaging studies.
Keywords: cortical areas; fMRI; functional connectivity; neonate; parcellation.
Conflict of interest statement
COMPETING INTERESTS Damien A. Fair is a patent holder on the Framewise Integrated Real-Time Motion Monitoring (FIRMM) software. He is also a co-founder of Turing Medical Inc. that licenses this software. The nature of this financial interest and the design of the study have been reviewed by the University of Minnesota, and a plan has been established to ensure that this research study is not affected by the financial interest. The other authors declare no competing interests.
Figures



References
-
- Felleman D. J. & Van Essen D. C. Distributed hierarchical processing in the primate cerebral cortex. Cereb. Cortex 1, 1–47 (1991). - PubMed
-
- Sejnowski T. J. & Churchland P. S. Brain and cognition. in Foundations of cognitive science, (pp (ed. Posner M. I.) vol. 888 301–356 (The MIT Press, xiv, 1989).
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
