This is a preprint.
Neuronal Glycogen Breakdown Mitigates Tauopathy via Pentose Phosphate Pathway-Mediated Oxidative Stress Reduction
- PMID: 37986935
- PMCID: PMC10659530
- DOI: 10.21203/rs.3.rs-3526342/v1
Neuronal Glycogen Breakdown Mitigates Tauopathy via Pentose Phosphate Pathway-Mediated Oxidative Stress Reduction
Update in
-
Neuronal glycogen breakdown mitigates tauopathy via pentose-phosphate-pathway-mediated oxidative stress reduction.Nat Metab. 2025 Jul;7(7):1375-1391. doi: 10.1038/s42255-025-01314-w. Epub 2025 Jun 30. Nat Metab. 2025. PMID: 40588539 Free PMC article.
Abstract
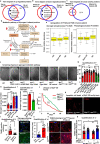
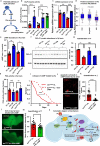
Tauopathies encompass a range of neurodegenerative disorders, such as Alzheimer's disease (AD) and frontotemporal dementia (FTD). Unfortunately, current treatment approaches for tauopathies have yielded limited success, underscoring the pressing need for novel therapeutic strategies. We observed distinct signatures of impaired glycogen metabolism in the Drosophila brain of the tauopathy model and the brain of AD patients, indicating a link between tauopathies and glycogen metabolism. We demonstrate that the breakdown of neuronal glycogen by activating glycogen phosphorylase (GlyP) ameliorates the tauopathy phenotypes in flies and induced pluripotent stem cell (iPSC) derived neurons from FTD patients. We observed that glycogen breakdown redirects the glucose flux to the pentose phosphate pathway to alleviate oxidative stress. Our findings uncover a critical role for increased GlyP activity in mediating the neuroprotection benefit of dietary restriction (DR) through the cAMP-mediated protein kinase A (PKA) activation. Our studies identify impaired glycogen metabolism as a key hallmark for tauopathies and offer a promising therapeutic target in tauopathy treatment.
Conflict of interest statement
Competing interests PK is a founder and a member of the scientific advisory board at Juvify Bio. Other authors have no conflicts of interest.
Figures


References
-
- Kovacs G. G. Tauopathies. Handb Clin Neurol 145, 355–368 (2017). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

