This is a preprint.
Fold-and-fuse neurulation in zebrafish requires Vangl2
- PMID: 37986956
- PMCID: PMC10659374
- DOI: 10.1101/2023.11.09.566412
Fold-and-fuse neurulation in zebrafish requires Vangl2
Update in
-
Fold-and-fuse neurulation in zebrafish requires vangl2.Dev Biol. 2025 Aug;524:55-68. doi: 10.1016/j.ydbio.2025.05.001. Epub 2025 May 5. Dev Biol. 2025. PMID: 40334836
Abstract
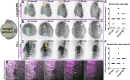
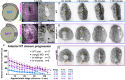
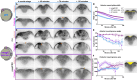
Shaping of the future brain and spinal cord during neurulation is an essential component of early vertebrate development. In amniote embryos, primary neurulation occurs through a "fold-and-fuse" mechanism by which the edges of the neural plate fuse into the hollow neural tube. Failure of neural fold fusion results in neural tube defects (NTDs), which are among the most devastating and common congenital anomalies worldwide. Unlike amniotes, the zebrafish neural tube develops largely via formation of a solid neural keel that later cavitates to form a midline lumen. Although many aspects of primary neurulation are conserved in zebrafish, including neural fold zippering, it was not clear how well these events resemble analogous processes in amniote embryos. Here, we demonstrate that despite outward differences, zebrafish anterior neurulation closely resembles that of mammals. For the first time in zebrafish embryos, we directly observe enclosure of a lumen by the bilateral neural folds, which fuse by zippering between at least two distinct closure sites. Both the apical constriction that elevates the neural folds and the zippering that fuses them coincide with apical Myosin enrichment. We further show that embryos lacking vangl2, a core planar cell polarity and NTD risk gene, exhibit delayed and abnormal neural fold fusion that fails to enclose a lumen. These defects can also be observed in fixed embryos, enabling their detection without live imaging. Together, our data provide direct evidence for fold-and-fuse neurulation in zebrafish and its disruption upon loss of an NTD risk gene, highlighting the deep conservation of primary neurulation across vertebrates.
Keywords: Neural tube defects; Neurulation; Planar cell polarity; Vangl2; Zebrafish.
Conflict of interest statement
Competing Interests The authors declare no competing interests.
Figures




References
-
- Parker S. E. et al. , Updated National Birth Prevalence estimates for selected birth defects in the United States, 2004–2006. Birth Defects Res A Clin Mol Teratol 88, 1008–1016 (2010). - PubMed
-
- Massarwa R., Ray H. J., Niswander L., Morphogenetic movements in the neural plate and neural tube: mouse. Wiley Interdiscip Rev Dev Biol 3, 59–68 (2014). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
