This is a preprint.
Tissue-resident alveolar macrophages reduce O3-induced inflammation via MerTK mediated efferocytosis
- PMID: 37986982
- PMCID: PMC10659406
- DOI: 10.1101/2023.11.06.565865
Tissue-resident alveolar macrophages reduce O3-induced inflammation via MerTK mediated efferocytosis
Update in
-
Tissue-Resident Alveolar Macrophages Reduce Ozone-induced Inflammation via MerTK-mediated Efferocytosis.Am J Respir Cell Mol Biol. 2024 Jun;70(6):493-506. doi: 10.1165/rcmb.2023-0390OC. Am J Respir Cell Mol Biol. 2024. PMID: 38386777 Free PMC article.
Abstract
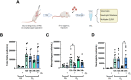
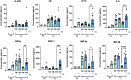
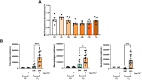
Lung inflammation, caused by acute exposure to ozone (O3) - one of the six criteria air pollutants - is a significant source of morbidity in susceptible individuals. Alveolar macrophages (AMØs) are the most abundant immune cells in the normal lung and their number increases following O3 exposure. However, the role of AMØs in promoting or limiting O3-induced lung inflammation has not been clearly defined. Here, we used a mouse model of acute O3 exposure, lineage tracing, genetic knockouts, and data from O3-exposed human volunteers to define the role and ontogeny of AMØs during acute O3 exposure. Lineage tracing experiments showed that 12, 24, and 72 h after exposure to O3 (2 ppm) for 3h all AMØs were tissue-resident origin. Similarly, in humans exposed to FA and O3 (200 ppb) for 135 minutes, we did not observe ~21h post-exposure an increase in monocyte-derived AMØs by flow cytometry. Highlighting a role for tissue-resident AMØs, we demonstrate that depletion of tissue-resident AMØs with clodronate-loaded liposomes led to persistence of neutrophils in the alveolar space after O3 exposure, suggesting that impaired neutrophil clearance (i.e., efferocytosis) leads to prolonged lung inflammation. Moreover, depletion of tissue-resident AMØ demonstrated reduced clearance of intratracheally instilled apoptotic Jurkat cells, consistent with reduced efferocytosis. Genetic ablation of MerTK - a key receptor involved in efferocytosis - also resulted in impaired clearance of apoptotic neutrophils followed O3 exposure. Overall, these findings underscore the pivotal role of tissue-resident AMØs in resolving O3-induced inflammation via MerTK-mediated efferocytosis.
Figures





References
-
- Fuller R. et al. Pollution and health: a progress update. Lancet Planet. Heal. 6, e535–e547 (2022). - PubMed
-
- Liao H., Chen W. T. & Seinfeld J. H. Role of climate change in global predictions of future tropospheric ozone and aerosols. J. Geophys. Res. Atmos. 111, (2006).
-
- Samet J. M. et al. The National Morbidity, Mortality, and Air Pollution Study. Part II: Morbidity and mortality from air pollution in the United States - PubMed. https://pubmed.ncbi.nlm.nih.gov/11354823/ (2000). - PubMed
Publication types
Grants and funding
- R01 ES034350/ES/NIEHS NIH HHS/United States
- R21 AG075423/AG/NIA NIH HHS/United States
- S10 OD011996/OD/NIH HHS/United States
- Z01 ES102005/ImNIH/Intramural NIH HHS/United States
- R01 HL153312/HL/NHLBI NIH HHS/United States
- P01 HL154998/HL/NHLBI NIH HHS/United States
- R01 ES028829/ES/NIEHS NIH HHS/United States
- R01 ES027574/ES/NIEHS NIH HHS/United States
- P01 AG049665/AG/NIA NIH HHS/United States
- U19 AI135964/AI/NIAID NIH HHS/United States
- T32 ES021432/ES/NIEHS NIH HHS/United States
- R01 HL158139/HL/NHLBI NIH HHS/United States
- P30 CA060553/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous
