Trends in the Development of Antibody-Drug Conjugates for Cancer Therapy
- PMID: 37987250
- PMCID: PMC10660735
- DOI: 10.3390/antib12040072
Trends in the Development of Antibody-Drug Conjugates for Cancer Therapy
Abstract
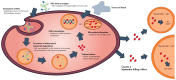
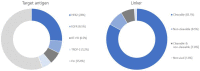
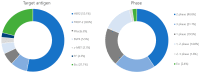
In cancer treatment, the first-generation, cytotoxic drugs, though effective against cancer cells, also harmed healthy ones. The second-generation targeted cancer cells precisely to inhibit their growth. Enter the third-generation, consisting of immuno-oncology drugs, designed to combat drug resistance and bolster the immune system's defenses. These advanced therapies operate by obstructing the uncontrolled growth and spread of cancer cells through the body, ultimately eliminating them effectively. Within the arsenal of cancer treatment, monoclonal antibodies offer several advantages, including inducing cancer cell apoptosis, precise targeting, prolonged presence in the body, and minimal side effects. A recent development in cancer therapy is Antibody-Drug Conjugates (ADCs), initially developed in the mid-20th century. The second generation of ADCs addressed this issue through innovative antibody modification techniques, such as DAR regulation, amino acid substitutions, incorporation of non-natural amino acids, and enzymatic drug attachment. Currently, a third generation of ADCs is in development. This study presents an overview of 12 available ADCs, reviews 71 recent research papers, and analyzes 128 clinical trial reports. The overarching objective is to gain insights into the prevailing trends in ADC research and development, with a particular focus on emerging frontiers like potential targets, linkers, and drug payloads within the realm of cancer treatment.
Keywords: antibody drug conjugates; conjugation; linker; monoclonal antibody; payload.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

