Characterization of neuromuscular transmission and projections of muscle motor neurons in the rat stomach
- PMID: 37987773
- PMCID: PMC11208016
- DOI: 10.1152/ajpgi.00194.2023
Characterization of neuromuscular transmission and projections of muscle motor neurons in the rat stomach
Abstract
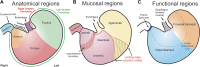
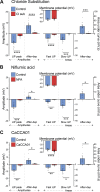
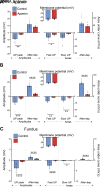
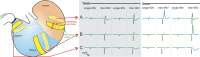
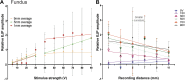
The stomach is the primary reservoir of the gastrointestinal tract, where ingested content is broken down into small particles. Coordinated relaxation and contraction is essential for rhythmic motility and digestion, but how the muscle motor innervation is organized to provide appropriate graded regional control is not established. In this study, we recorded neuromuscular transmission to the circular muscle using intracellular microelectrodes to investigate the spread of the influence of intrinsic motor neurons. In addition, microanatomical investigations of neuronal projections and pharmacological analysis were conducted to investigate neuromuscular relationships. We found that inhibitory neurotransmission to the circular muscle is graded with stimulus strength and circumferential distance from the stimulation site. The influence of inhibitory neurons declined between 1 and 11 mm from the stimulation site. In the antrum, corpus, and fundus, the declines at 11 mm were about 20%, 30%, and 50%, respectively. Stimulation of inhibitory neurons elicited biphasic hyperpolarizing potentials often followed by prolonged depolarizing events in the distal stomach, but only hyperpolarizing events in the proximal stomach. Excitatory neurotransmission influence varied greatly between proximal stomach, where depolarizing events occurred, and distal stomach, where no direct electrical effects in the muscle were observed. Structural studies using microlesion surgeries confirmed a dominant circumferential projection. We conclude that motor neuron influences extend around the gastric circumference, that the effectiveness can be graded by the recruitment of different numbers of motor neuron nerve terminals to finely control gastric motility, and that the ways in which the neurons influence the muscle differ between anatomical regions.NEW & NOTEWORTHY This study provides a detailed mapping of nerve transmission to the circular muscle of the different anatomical regions of rat stomach. It shows that excitatory and inhibitory influences extend around the gastric circumference and that there is a summation of neural influence that allows for finely graded control of muscle tension and length. Nerve-mediated electrical events are qualitatively and quantitatively different between regions, for example, excitatory neurons have direct effects on fundus but not antral muscle.
Keywords: enteric motor neuron; intracellular electrophysiology; neuromuscular junction; smooth muscle; stomach.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures







Similar articles
-
Distension-evoked ascending and descending reflexes in the isolated guinea-pig stomach.J Auton Nerv Syst. 1997 Jan 12;62(1-2):94-102. doi: 10.1016/s0165-1838(96)00115-4. J Auton Nerv Syst. 1997. PMID: 9021655
-
Loss of enteric motor neurotransmission in the gastric fundus of Sl/Sl(d) mice.J Physiol. 2002 Sep 15;543(Pt 3):871-87. doi: 10.1113/jphysiol.2002.021915. J Physiol. 2002. PMID: 12231645 Free PMC article.
-
Interstitial cells of Cajal: primary targets of enteric motor innervation.Anat Rec. 2001 Jan 1;262(1):125-35. doi: 10.1002/1097-0185(20010101)262:1<125::AID-AR1017>3.0.CO;2-I. Anat Rec. 2001. PMID: 11146435 Review.
-
Optogenetic analysis of neuromuscular transmission in the colon of ChAT-ChR2-YFP BAC transgenic mice.Am J Physiol Gastrointest Liver Physiol. 2019 Nov 1;317(5):G569-G579. doi: 10.1152/ajpgi.00089.2019. Epub 2019 Aug 14. Am J Physiol Gastrointest Liver Physiol. 2019. PMID: 31411893 Free PMC article.
-
Enteric pathways in the stomach.Anat Rec. 2001 Jan 1;262(1):47-57. doi: 10.1002/1097-0185(20010101)262:1<47::AID-AR1010>3.0.CO;2-1. Anat Rec. 2001. PMID: 11146428 Review.
Cited by
-
Surface mapping of gastric motor functions using MRI: a comparative study between humans and rats.Am J Physiol Gastrointest Liver Physiol. 2024 Sep 1;327(3):G345-G359. doi: 10.1152/ajpgi.00045.2024. Epub 2024 Jun 25. Am J Physiol Gastrointest Liver Physiol. 2024. PMID: 38915290 Free PMC article.
-
Physical Exercise as a Therapeutic Approach in Gastrointestinal Diseases.J Clin Med. 2025 Mar 3;14(5):1708. doi: 10.3390/jcm14051708. J Clin Med. 2025. PMID: 40095789 Free PMC article. Review.
-
Mapping the rat gastric slow-wave conduction pathway: bridging in vitro and in vivo methods, revealing a loosely coupled region in the distal stomach.Am J Physiol Gastrointest Liver Physiol. 2024 Aug 1;327(2):G254-G266. doi: 10.1152/ajpgi.00069.2024. Epub 2024 Jun 11. Am J Physiol Gastrointest Liver Physiol. 2024. PMID: 38860855 Free PMC article.
-
Acute Cervical Vagus Nerve Stimulation Modulates Gastric Slow Waves in the Distal Rat Stomach.Neurogastroenterol Motil. 2025 Jul;37(7):e70030. doi: 10.1111/nmo.70030. Epub 2025 Mar 27. Neurogastroenterol Motil. 2025. PMID: 40145403 Free PMC article.
References
-
- Cannon WB. The movements of the stomach studied by means of the Roentgen rays. Am J Physiol 1: 359–382, 1898. doi:10.1152/ajplegacy.1898.1.3.359. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

