Enzymatic vitamin A2 production enables red-shifted optogenetics
- PMID: 37987804
- PMCID: PMC10730639
- DOI: 10.1007/s00424-023-02880-2
Enzymatic vitamin A2 production enables red-shifted optogenetics
Abstract
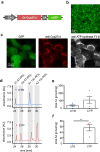
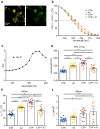
Optogenetics is a technology using light-sensitive proteins to control signaling pathways and physiological processes in cells and organs and has been applied in neuroscience, cardiovascular sciences, and many other research fields. Most commonly used optogenetic actuators are sensitive to blue and green light, but red-light activation would allow better tissue penetration and less phototoxicity. Cyp27c1 is a recently deorphanized cytochrome P450 enzyme that converts vitamin A1 to vitamin A2, thereby red-shifting the spectral sensitivity of visual pigments and enabling near-infrared vision in some aquatic species.Here, we investigated the ability of Cyp27c1-generated vitamin A2 to induce a shift in spectral sensitivity of the light-gated ion channel Channelrhodopsin-2 (ChR2) and its red-shifted homolog ReaChR. We used patch clamp to measure photocurrents at specific wavelengths in HEK 293 cells expressing ChR2 or ReaChR. Vitamin A2 incubation red-shifted the wavelength for half-maximal currents (λ50%) by 6.8 nm for ChR2 and 12.4 nm for ReaChR. Overexpression of Cyp27c1 in HEK 293 cells showed mitochondrial localization, and HPLC analysis showed conversion of vitamin A1 to vitamin A2. Notably, the λ50% of ChR2 photocurrents was red-shifted by 10.5 nm, and normalized photocurrents at 550 nm were about twofold larger with Cyp27c1 expression. Similarly, Cyp27c1 shifted the λ50% of ReaChR photocurrents by 14.3 nm and increased normalized photocurrents at 650 nm almost threefold.Since vitamin A2 incubation is not a realistic option for in vivo applications and expression of Cyp27c1 leads to a greater red-shift in spectral sensitivity, we propose co-expression of this enzyme as a novel strategy for red-shifted optogenetics.
Keywords: ChR2; Cyp27c1; Optogenetics; ReaChR; Vitamin A2.
© 2023. The Author(s).
Conflict of interest statement
JCC holds a US patent for the use of Cyp27c1 co-expression to red-shift optogenetic actuators (United States Patent 10047130). The authors have no other financial or non-financial competing interests to disclose.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

