Lactate- and immunomagnetic-purified hiPSC-derived cardiomyocytes generate comparable engineered cardiac tissue constructs
- PMID: 37988170
- PMCID: PMC10906451
- DOI: 10.1172/jci.insight.172168
Lactate- and immunomagnetic-purified hiPSC-derived cardiomyocytes generate comparable engineered cardiac tissue constructs
Abstract
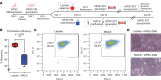
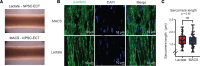
Three-dimensional engineered cardiac tissue (ECT) using purified human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) has emerged as an appealing model system for the study of human cardiac biology and disease. A recent study reported widely used metabolic (lactate) purification of monolayer hiPSC-CM cultures results in an ischemic cardiomyopathy-like phenotype compared with magnetic antibody-based cell sorting (MACS) purification, complicating the interpretation of studies using lactate-purified hiPSC-CMs. Herein, our objective was to determine if use of lactate relative to MACS-purified hiPSC-CMs affects the properties of resulting hiPSC-ECTs. Therefore, hiPSC-CMs were differentiated and purified using either lactate-based media or MACS. Global proteomics revealed that lactate-purified hiPSC-CMs displayed a differential phenotype over MACS hiPSC-CMs. hiPSC-CMs were then integrated into 3D hiPSC-ECTs and cultured for 4 weeks. Structurally, there was no significant difference in sarcomere length between lactate and MACS hiPSC-ECTs. Assessment of isometric twitch force and Ca2+ transient measurements revealed similar functional performance between purification methods. High-resolution mass spectrometry-based quantitative proteomics showed no significant difference in protein pathway expression or myofilament proteoforms. Taken together, this study demonstrates that lactate- and MACS-purified hiPSC-CMs generate ECTs with comparable structural, functional, and proteomic features, and it suggests that lactate purification does not result in an irreversible change in a hiPSC-CM phenotype.
Keywords: Cardiology; Cardiovascular disease; Stem cells; iPS cells.
Conflict of interest statement
Figures





Update of
-
Lactate and Immunomagnetic-purified iPSC-derived Cardiomyocytes Generate Comparable Engineered Cardiac Tissue Constructs.bioRxiv [Preprint]. 2023 May 6:2023.05.05.539642. doi: 10.1101/2023.05.05.539642. bioRxiv. 2023. Update in: JCI Insight. 2024 Jan 9;9(1):e172168. doi: 10.1172/jci.insight.172168. PMID: 37205556 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

