Thrombotic microangiopathy following systemic AAV administration is dependent on anti-capsid antibodies
- PMID: 37988172
- PMCID: PMC10760971
- DOI: 10.1172/JCI173510
Thrombotic microangiopathy following systemic AAV administration is dependent on anti-capsid antibodies
Abstract
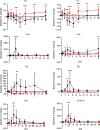
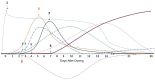
BACKGROUNDSystemic administration of adeno-associated virus (AAV) can trigger life-threatening inflammatory responses, including thrombotic microangiopathy (TMA), acute kidney injury due to atypical hemolytic uremic syndrome-like complement activation, immune-mediated myocardial inflammation, and hepatic toxicity.METHODSWe describe the kinetics of immune activation following systemic AAV serotype 9 (AAV9) administration in 38 individuals following 2 distinct prophylactic immunomodulation regimens. Group 1 received corticosteroids and Group 2 received rituximab plus sirolimus in addition to steroids to prevent anti-AAV antibody formation.RESULTSGroup 1 participants had a rapid increase in immunoglobulin M (IgM) and IgG. Increase in D-dimer, decline in platelet count, and complement activation are indicative of TMA. All Group 1 participants demonstrated activation of both classical and alternative complement pathways, as indicated by depleted C4 and elevated soluble C5b-9, Ba, and Bb antigens. Group 2 patients did not have a significant change in IgM or IgG and had minimal complement activation.CONCLUSIONSThis study demonstrates that TMA in the setting of AAV gene therapy is antibody dependent (classical pathway) and amplified by the alternative complement pathway. Critical time points and interventions are identified to allow for management of immune-mediated events that impact the safety and efficacy of systemic gene therapy.
Keywords: Genetics; Immunology; Innate immunity.
Figures



Comment in
-
Improving the safety of systemic viral gene therapy.J Clin Invest. 2024 Jan 2;134(1):e177078. doi: 10.1172/JCI177078. J Clin Invest. 2024. PMID: 38165031 Free PMC article. No abstract available.
References
-
- Sherafat R. Toxicity risks of adeno-associated virus (AAV) vectors for gene therapy (GT). Paper presented at: FDA Cellular, Tissue, and Gene Therapies Advisory Committee (CTGTAC) Meeting #70; September 2–3, 2021; Virtual. https://www.fda.gov/media/151969/download Accessed November 1, 2023.
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

