Sex-specific modulation of amyloid-β on tau phosphorylation underlies faster tangle accumulation in females
- PMID: 37988283
- PMCID: PMC10994548
- DOI: 10.1093/brain/awad397
Sex-specific modulation of amyloid-β on tau phosphorylation underlies faster tangle accumulation in females
Erratum in
-
Correction to: Sex-specific modulation of amyloid-β on tau phosphorylation underlies faster tangle accumulation in females.Brain. 2025 Jun 3;148(6):e55. doi: 10.1093/brain/awaf174. Brain. 2025. PMID: 40397503 Free PMC article. No abstract available.
Abstract
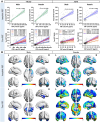
Females are disproportionately affected by dementia due to Alzheimer's disease. Despite a similar amyloid-β (Aβ) load, a higher load of neurofibrillary tangles (NFTs) is seen in females than males. Previous literature has proposed that Aβ and phosphorylated-tau (p-tau) synergism accelerates tau tangle formation, yet the effect of biological sex in this process has been overlooked. In this observational study, we examined longitudinal neuroimaging data from the TRIAD and ADNI cohorts from Canada and USA, respectively. We assessed 457 participants across the clinical spectrum of Alzheimer's disease. All participants underwent baseline multimodal imaging assessment, including MRI and PET, with radioligands targeting Aβ plaques and tau tangles, respectively. CSF data were also collected. Follow-up imaging assessments were conducted at 1- and 2-year intervals for the TRIAD cohort and 1-, 2- and 4-year intervals for the ADNI cohort. The upstream pathological events contributing to faster tau progression in females were investigated-specifically, whether the contribution of Aβ and p-tau synergism to accelerated tau tangle formation is modulated by biological sex. We hypothesized that cortical Aβ predisposes tau phosphorylation and tangle accumulation in a sex-specific manner. Findings revealed that Aβ-positive females presented higher CSF p-tau181 concentrations compared with Aβ-positive males in both the TRIAD (P = 0.04, Cohen's d = 0.51) and ADNI (P = 0.027, Cohen's d = 0.41) cohorts. In addition, Aβ-positive females presented faster NFT accumulation compared with their male counterparts (TRIAD: P = 0.026, Cohen's d = 0.52; ADNI: P = 0.049, Cohen's d = 1.14). Finally, the triple interaction between female sex, Aβ and CSF p-tau181 was revealed as a significant predictor of accelerated tau accumulation at the 2-year follow-up visit (Braak I: P = 0.0067, t = 2.81; Braak III: P = 0.017, t = 2.45; Braak IV: P = 0.002, t = 3.17; Braak V: P = 0.006, t = 2.88; Braak VI: P = 0.0049, t = 2.93). Overall, we report sex-specific modulation of cortical Aβ in tau phosphorylation, consequently facilitating faster NFT progression in female individuals over time. This presents important clinical implications and suggests that early intervention that targets Aβ plaques and tau phosphorylation may be a promising therapeutic strategy in females to prevent the further accumulation and spread of tau aggregates.
Keywords: Alzheimer's disease; amyloid-β; sex difference; tau neurofibrillary tangle progression; tau phsophorylation.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
Conflict of interest statement
The authors report no competing interests related to this work. Outside the work presented in this paper, P.R.N. provides consultancy services for Roche, Cerveau Radiopharmaceuticals, Lilly, Eisai, Pfizer and Novo Nordisk. He also serves as a clinical trials investigator for Biogen, Novo Nordisk. S.G. is a member of the scientific advisory boards of Alzheon, AmyriAD, Eisai Canada, Enigma USA, Lilly Canada, Medesis, Okutsa Canada, Roche Canada and TauRx. He is a member of the editorial board of JPAD and of the Neurotorium. He has given lectures under the auspices of Biogen Canada and Lundbeck Korea. H.Z. has served on scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZPath, Annexon, Apellis, Artery Therapeutics, AZTherapies, CogRx, Denali, Eisai, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics and Wave, has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure, Biogen and Roche, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). K.B. has served as a consultant and on advisory boards for Acumen, ALZPath, BioArctic, Biogen, Eisai, Julius Clinical, Lilly, Novartis, Ono Pharma, Prothena, Roche Diagnostics and Siemens Healthineers; has served at data monitoring committees for Julius Clinical and Novartis; has given lectures, produced educational materials and participated in educational programs for Biogen, Eisai and Roche Diagnostics; and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program.
Figures


References
-
- Masters CL, Bateman R, Blennow K, Rowe CC, Sperling RA, Cummings JL. Alzheimer’s disease. Nat Rev Dis Primers. 2015;1:15056. - PubMed
-
- Chien DT, Bahri S, Szardenings AK, et al. Early clinical PET imaging results with the novel PHF-tau radioligand [F-18]-T807. J Alzheimers Dis. 2013;34:457–468. - PubMed
-
- Chien DT, Szardenings AK, Bahri S, et al. Early clinical PET imaging results with the novel PHF-tau radioligand [F18]-T808. J Alzheimers Dis. 2014;38:171–184. - PubMed
-
- Okamura N, Furumoto S, Fodero-Tavoletti MT, et al. Non-invasive assessment of Alzheimer’s disease neurofibrillary pathology using 18F-THK5105 PET. Brain. 2014;137(Pt 6):1762–1771. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

