Functional ultrasound imaging of stroke in awake rats
- PMID: 37988288
- PMCID: PMC10662948
- DOI: 10.7554/eLife.88919
Functional ultrasound imaging of stroke in awake rats
Abstract
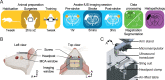
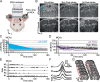
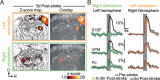
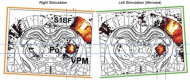
Anesthesia is a major confounding factor in preclinical stroke research as stroke rarely occurs in sedated patients. Moreover, anesthesia affects both brain functions and the stroke outcome acting as neurotoxic or protective agents. So far, no approaches were well suited to induce stroke while imaging hemodynamics along with simultaneous large-scale recording of brain functions in awake animals. For this reason, the first critical hours following the stroke insult and associated functional alteration remain poorly understood. Here, we present a strategy to investigate both stroke hemodynamics and stroke-induced functional alterations without the confounding effect of anesthesia, i.e., under awake condition. Functional ultrasound (fUS) imaging was used to continuously monitor variations in cerebral blood volume (CBV) in +65 brain regions/hemispheres for up to 3 hr after stroke onset. The focal cortical ischemia was induced using a chemo-thrombotic agent suited for permanent middle cerebral artery occlusion in awake rats and followed by ipsi- and contralesional whiskers stimulation to investigate on the dynamic of the thalamocortical functions. Early (0-3 hr) and delayed (day 5) fUS recording enabled to characterize the features of the ischemia (location, CBV loss), spreading depolarizations (occurrence, amplitude) and functional alteration of the somatosensory thalamocortical circuits. Post-stroke thalamocortical functions were affected at both early and later time points (0-3 hr and 5 days) after stroke. Overall, our procedure facilitates early, continuous, and chronic assessments of hemodynamics and cerebral functions. When integrated with stroke studies or other pathological analyses, this approach seeks to enhance our comprehension of physiopathologies towards the development of pertinent therapeutic interventions.
Keywords: awake rat; functional diaschisis; functional ultrasound imaging; ischemic stroke; neuroscience; rat; thalamocortical circuit.
© 2023, Brunner et al.
Conflict of interest statement
CB, GM, AU No competing interests declared
Figures






Update of
- doi: 10.1101/2023.05.31.543179
- doi: 10.7554/eLife.88919.1
- doi: 10.7554/eLife.88919.2
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

