Long non-coding RNA SNHG17 may function as a competitive endogenous RNA in diffuse large B-cell lymphoma progression by sponging miR-34a-5p
- PMID: 37988356
- PMCID: PMC10662735
- DOI: 10.1371/journal.pone.0294729
Long non-coding RNA SNHG17 may function as a competitive endogenous RNA in diffuse large B-cell lymphoma progression by sponging miR-34a-5p
Erratum in
-
Correction: Long non-coding RNA SNHG17 may function as a competitive endogenous RNA in diffuse large B-cell lymphoma progression by sponging miR-34a-5p.PLoS One. 2024 Dec 31;19(12):e0317025. doi: 10.1371/journal.pone.0317025. eCollection 2024. PLoS One. 2024. PMID: 39739982 Free PMC article.
Abstract
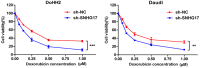
We investigated the functional mechanism of long non-coding small nucleolar host gene 17 (SNHG17) in diffuse large B-cell lymphoma (DLBCL). lncRNAs related to the prognosis of patients with DLBCL were screened to analyze long non-coding small nucleolar host gene 17 (SNHG17) expression in DLBCL and normal tissues, and a nomogram established for predicting DLBCL prognosis. SNHG17 expression in B-cell lymphoma cells was detected using qPCR. The effects of SNHG17 with/without doxorubicin on the proliferation and apoptosis of DoHH2 and Daudi were detected. The effects of combined SNHG17 and doxorubicin were analyzed. The regulatory function of SNHG17 in DLBCL was investigated using a mouse tumor xenotransplantation model. RNA sequencing was used to analyze the signaling pathways involved in SNHG17 knockdown in B-cell lymphoma cell lines. The target relationships among SNHG17, microRNA, and downstream mRNA biomolecules were detected. A higher SNHG17 level predicted a lower survival rate. SNHG17 was highly expressed in DLBCL patient tissues and cell lines. We established a prognostic model containing SNHG17 expression, which could effectively predict the overall survival rate of DLBCL patients. SNHG17 knockdown inhibited the proliferation and induced the apoptosis of B-cell lymphoma cells, and the combination of SNHG17 and doxorubicin had a synergistic effect. SNHG17, miR-34a-5p, and ZESTE gene enhancer homolog 2 (EZH2) had common hypothetical binding sites, and the luciferase reporter assay verified that miR-34a-5p was the direct target of SNHG17, and EZH2 was the direct target of miR-34a-5p. The carcinogenic function of SNHG17 in the proliferation and apoptosis of DLBCL cells was partially reversed by a miR-34a-5p inhibitor. SNHG17 increases EZH2 levels by inhibiting miR-34a-5p. Our findings indicate SNHG17 as critical for promoting DLBCL progression by regulating the EZH2 signaling pathway and sponging miR-34a-5p. These findings provide a new prognostic marker and therapeutic target for the prognosis and treatment of DLBCL.
Copyright: © 2023 Lu et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Kaatsch P., Epidemiology of childhood cancer. Cancer Treatment Reviews, 2010. 36(4). - PubMed
-
- R, P.J. and C.A. M, The emergence of lncRNAs in cancer biology. Cancer discovery, 2011. 1(5). doi: 10.1158/2159-8290.CD-11-0209 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

