Feasibility of Coronary Access Following Redo-TAVR for Evolut Failure: A Computed Tomography Simulation Study
- PMID: 37988439
- PMCID: PMC10653288
- DOI: 10.1161/CIRCINTERVENTIONS.123.013238
Feasibility of Coronary Access Following Redo-TAVR for Evolut Failure: A Computed Tomography Simulation Study
Abstract
Background: Coronary accessibility following redo-transcatheter aortic valve replacement (redo-TAVR) is increasingly important, particularly in younger low-risk patients. This study aimed to predict coronary accessibility after simulated Sapien-3 balloon-expandable valve implantation within an Evolut supra-annular, self-expanding valve using pre-TAVR computed tomography (CT) imaging.
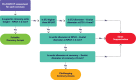
Methods: A total of 219 pre-TAVR CT scans from the Evolut Low-Risk CT substudy were analyzed. Virtual Evolut and Sapien-3 valves were sized using CT-based diameters. Two initial Evolut implant depths were analyzed, 3 and 5 mm. Coronary accessibility was evaluated for 2 Sapien-3 in Evolut implant positions: Sapien-3 outflow at Evolut node 4 and Evolut node 5.
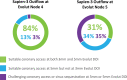
Results: With a 3-mm initial Evolut implant depth, suitable coronary access was predicted in 84% of patients with the Sapien-3 outflow at Evolut node 4, and in 31% of cases with the Sapien-3 outflow at Evolut node 5 (P<0.001). Coronary accessibility improved with a 5-mm Evolut implant depth: 97% at node 4 and 65% at node 5 (P<0.001). When comparing 3- to 5-mm Evolut implant depth, sinus sequestration was the lowest with Sapien-3 outflow at Evolut node 4 (13% versus 2%; P<0.001), and the highest at Evolut node 5 (61% versus 32%; P<0.001).
Conclusions: Coronary accessibility after Sapien-3 in Evolut redo-TAVR relates to the initial Evolut implant depth, the Sapien-3 outflow position within the Evolut, and the native annular anatomy. This CT-based quantitative analysis may provide useful information to inform and refine individualized preprocedural CT planning of the initial TAVR and guide lifetime management for future coronary access after redo-TAVR.
Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT02701283.
Keywords: balloon-expandable valve; coronary access; implant depth; self-expanding valve; transcatheter aortic valve replacement.
Conflict of interest statement
Figures


Comment in
-
CT Simulation for Redo-TAVR: Can We Predict Future Coronary Access?Circ Cardiovasc Interv. 2023 Nov;16(11):e013572. doi: 10.1161/CIRCINTERVENTIONS.123.013572. Epub 2023 Nov 21. Circ Cardiovasc Interv. 2023. PMID: 37988441 No abstract available.
References
-
- Akodad M, Sellers S, Gulsin GS, Tzimas G, Landes U, Chatfield AG, Chuang A, Meier D, Leipsic J, Blanke P, et al. . Leaflet and neoskirt height in transcatheter heart valves: implications for repeat procedures and coronary access. JACC Cardiovasc Interv. 2021;14:2298–2300. doi: 10.1016/j.jcin.2021.07.034 - PubMed
-
- Landes U, Webb JG, De Backer O, Sondergaard L, Abdel-Wahab M, Crusius L, Kim WK, Hamm C, Buzzatti N, Montorfano M, et al. . Repeat transcatheter aortic valve replacement for transcatheter prosthesis dysfunction. J Am Coll Cardiol. 2020;75:1882–1893. doi: 10.1016/j.jacc.2020.02.051 - PubMed
-
- Ochiai T, Oakley L, Sekhon N, Komatsu I, Flint N, Kaewkes D, Yoon SH, Raschpichler M, Patel V, Tiwana R, et al. . Risk of coronary obstruction due to sinus sequestration in redo transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2020;13:2617–2627. doi: 10.1016/j.jcin.2020.09.022 - PubMed
-
- Tang GHL, Zaid S, Gupta E, Ahmad H, Khan A, Kovacic JC, Lansman SL, Dangas GD, Sharma SK, Kini A. Feasibility of repeat TAVR after SAPIEN 3 TAVR: a novel classification scheme and pilot angiographic study. JACC Cardiovasc Interv. 2019;12:1290–1292. doi: 10.1016/j.jcin.2019.02.020 - PubMed
-
- Yerasi C, Rogers T, Forrestal BJ, Case BC, Khan JM, Ben-Dor I, Satler LF, Garcia-Garcia HM, Cohen JE, Kitahara H, et al. . Transcatheter versus surgical aortic valve replacement in young, low-risk patients with severe aortic stenosis. JACC Cardiovasc Interv. 2021;14:1169–1180. doi: 10.1016/j.jcin.2021.03.058 - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

