Maternal serum levels of prokineticin-1 related to pregnancy complications and metformin use in women with polycystic ovary syndrome: a post hoc analysis of two prospective, randomised, placebo-controlled trials
- PMID: 37989369
- PMCID: PMC10668301
- DOI: 10.1136/bmjopen-2023-073619
Maternal serum levels of prokineticin-1 related to pregnancy complications and metformin use in women with polycystic ovary syndrome: a post hoc analysis of two prospective, randomised, placebo-controlled trials
Abstract
Objective: Serum prokineticin-1 (s-PROK1) in the second and third trimester of pregnancy is positively correlated to preeclampsia, intrauterine growth restriction (IUGR) and preterm delivery. Women with polycystic ovary syndrome (PCOS) are prone to these adverse pregnancy outcomes. However, the contribution of PROK1 to the development of pregnancy complications and the effect of metformin and hyperandrogenism on s-PROK1 in PCOS have not been studied previously.
Design: This work is a post hoc analysis of two prospective, randomised, placebo-controlled trials.
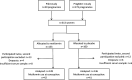
Setting: Pregnant women with PCOS were included from 11 study centres in Norway.
Participants: From 313 women, 264 participated in the present study after exclusions due to dropouts or insufficient serum samples.
Intervention: Women with PCOS were randomly administered with metformin or placebo, from first trimester to delivery.
Primary and secondary outcome measures: s-PROK1 was analysed using ELISA at gestational week 19 and related to pregnancy complications, fasting insulin levels, homoeostatic model assessment for insulin resistance (HOMA-IR), testosterone, or androstenedione levels, metformin use, PCOS phenotype and hyperandrogenism.
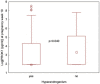
Results: Maternal s-PROK1 in the second trimester did not predict pregnancy-induced hypertension, pre-eclampsia or late miscarriage/preterm delivery in women with PCOS. However, s-PROK1 was lower in women who used metformin before inclusion, both in those randomised to metformin and to placebo, compared with those who did not. s-PROK1 was also lower in those who used metformin both at conception and during pregnancy compared with those who used metformin from inclusion or did not use metformin at all. s-PROK1 was lower in hyperandrogenic compared with normo-androgenic women with PCOS.
Conclusions: Maternal s-PROK1 in the second trimester did not predict pregnancy complications in PCOS. Those who used metformin at conception and/or during pregnancy had lower s-PROK1. PCOS women with hyperandrogenism exhibited lower s-PROK1 compared with normo-adrogenic phenotypes.
Trial registration number: NCT03259919 and NCT00159536.
Keywords: hypertension; maternal medicine; reproductive medicine; subfertility.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures


References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
