How Cell-Penetrating Peptides Behave Differently from Pore-Forming Peptides: Structure and Stability of Induced Transmembrane Pores
- PMID: 37989570
- PMCID: PMC11870675
- DOI: 10.1021/jacs.3c08014
How Cell-Penetrating Peptides Behave Differently from Pore-Forming Peptides: Structure and Stability of Induced Transmembrane Pores
Abstract
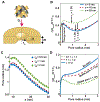
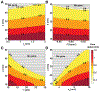
Peptide-induced transmembrane pore formation is commonplace in biology. Examples of transmembrane pores include pores formed by antimicrobial peptides (AMPs) and cell-penetrating peptides (CPPs) in bacterial membranes and eukaryotic membranes, respectively. In general, however, transmembrane pore formation depends on peptide sequences, lipid compositions, and intensive thermodynamic variables and is difficult to observe directly under realistic solution conditions, with structures that are challenging to measure directly. In contrast, the structure and phase behavior of peptide-lipid systems are relatively straightforward to map out experimentally for a broad range of conditions. Cubic phases are often observed in systems involving pore-forming peptides; however, it is not clear how the structural tendency to induce negative Gaussian curvature (NGC) in such phases is quantitatively related to the geometry of biological pores. Here, we leverage the theory of anisotropic inclusions and devise a facile method to estimate transmembrane pore sizes from geometric parameters of cubic phases measured from small-angle X-ray scattering (SAXS) and show that such estimates compare well with known pore sizes. Moreover, our model suggests that although AMPs can induce stable transmembrane pores for membranes with a broad range of conditions, pores formed by CPPs are highly labile, consistent with atomistic simulations.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
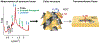
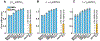

Update of
-
How cell penetrating peptides behave differently from pore forming peptides: structure and stability of induced transmembrane pores.bioRxiv [Preprint]. 2023 Jul 27:2023.07.26.550729. doi: 10.1101/2023.07.26.550729. bioRxiv. 2023. Update in: J Am Chem Soc. 2023 Dec 6;145(48):26095-26105. doi: 10.1021/jacs.3c08014. PMID: 37546874 Free PMC article. Updated. Preprint.
References
-
- Zasloff M Antimicrobial Peptides of Multicellular Organisms. Nature 2002, 415 (6870), 389–395. - PubMed
-
- Brogden KA Antimicrobial Peptides: Pore Formers or Metabolic Inhibitors in Bacteria? Nat. Rev. Microbiol 2005, 3 (3), 238–250. - PubMed
-
- Yu Y; Vroman JA; Bae SC; Granick S Vesicle Budding Induced by a Pore-Forming Peptide. J. Am. Chem. Soc 2010, 132 (1), 195–201. - PubMed
-
- Shai Y Mechanism of the Binding, Insertion and Destabilization of Phospholipid Bilayer Membranes by α-Helical Antimicrobial and Cell Non-Selective Membrane-Lytic Peptides. Biochim. Biophys. Acta, Biomembr 1999, 1462 (1), 55–70. - PubMed
-
- Youle RJ; Strasser A The BCL-2 Protein Family: Opposing Activities That Mediate Cell Death. Nat. Rev. Mol. Cell Biol 2008, 9 (1), 47–59. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

