CaV3.1 channels facilitate calcium wave generation and myogenic tone development in mouse mesenteric arteries
- PMID: 37989780
- PMCID: PMC10663617
- DOI: 10.1038/s41598-023-47715-3
CaV3.1 channels facilitate calcium wave generation and myogenic tone development in mouse mesenteric arteries
Abstract
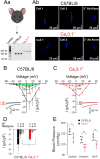
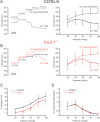
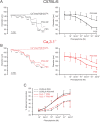
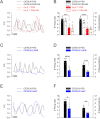
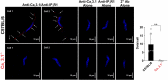
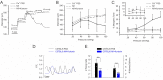
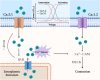
The arterial myogenic response to intraluminal pressure elicits constriction to maintain tissue perfusion. Smooth muscle [Ca2+] is a key determinant of constriction, tied to L-type (CaV1.2) Ca2+ channels. While important, other Ca2+ channels, particularly T-type could contribute to pressure regulation within defined voltage ranges. This study examined the role of one T-type Ca2+ channel (CaV3.1) using C57BL/6 wild type and CaV3.1-/- mice. Patch-clamp electrophysiology, pressure myography, blood pressure and Ca2+ imaging defined the CaV3.1-/- phenotype relative to C57BL/6. CaV3.1-/- mice had absent CaV3.1 expression and whole-cell current, coinciding with lower blood pressure and reduced mesenteric artery myogenic tone, particularly at lower pressures (20-60 mmHg) where membrane potential is hyperpolarized. This reduction coincided with diminished Ca2+ wave generation, asynchronous events of Ca2+ release from the sarcoplasmic reticulum, insensitive to L-type Ca2+ channel blockade (Nifedipine, 0.3 µM). Proximity ligation assay (PLA) confirmed IP3R1/CaV3.1 close physical association. IP3R blockade (2-APB, 50 µM or xestospongin C, 3 µM) in nifedipine-treated C57BL/6 arteries rendered a CaV3.1-/- contractile phenotype. Findings indicate that Ca2+ influx through CaV3.1 contributes to myogenic tone at hyperpolarized voltages through Ca2+-induced Ca2+ release tied to the sarcoplasmic reticulum. This study helps establish CaV3.1 as a potential therapeutic target to control blood pressure.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Genetic ablation of CaV3.2 channels enhances the arterial myogenic response by modulating the RyR-BKCa axis.Arterioscler Thromb Vasc Biol. 2015 Aug;35(8):1843-51. doi: 10.1161/ATVBAHA.115.305736. Epub 2015 Jun 11. Arterioscler Thromb Vasc Biol. 2015. PMID: 26069238 Free PMC article.
-
Caveolae Link CaV3.2 Channels to BKCa-Mediated Feedback in Vascular Smooth Muscle.Arterioscler Thromb Vasc Biol. 2018 Oct;38(10):2371-2381. doi: 10.1161/ATVBAHA.118.311394. Arterioscler Thromb Vasc Biol. 2018. PMID: 30354206
-
Ca(V)3.2 channels and the induction of negative feedback in cerebral arteries.Circ Res. 2014 Sep 12;115(7):650-61. doi: 10.1161/CIRCRESAHA.114.304056. Epub 2014 Aug 1. Circ Res. 2014. PMID: 25085940 Free PMC article.
-
Elementary calcium signaling in arterial smooth muscle.Channels (Austin). 2019 Dec;13(1):505-519. doi: 10.1080/19336950.2019.1688910. Channels (Austin). 2019. PMID: 31797713 Free PMC article. Review.
-
Aging, calcium channel signaling and vascular tone.Mech Ageing Dev. 2020 Oct;191:111336. doi: 10.1016/j.mad.2020.111336. Epub 2020 Sep 9. Mech Ageing Dev. 2020. PMID: 32918949 Free PMC article. Review.
Cited by
-
T-Type Voltage-Gated Calcium Channels: Potential Regulators of Smooth Muscle Contractility.Int J Mol Sci. 2024 Nov 19;25(22):12420. doi: 10.3390/ijms252212420. Int J Mol Sci. 2024. PMID: 39596484 Free PMC article. Review.
References
-
- Snutch TP, Peloquin J, Mathews E, McRory JE. Voltage-Gated Calcium Channels. Springer; 2005. pp. 61–94.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

