Brain metastasis, EGFR mutation subtype and generation of EGFR-TKI jointly influence the treatment outcome of patient with EGFR-mutant NSCLC
- PMID: 37989860
- PMCID: PMC10663477
- DOI: 10.1038/s41598-023-45815-8
Brain metastasis, EGFR mutation subtype and generation of EGFR-TKI jointly influence the treatment outcome of patient with EGFR-mutant NSCLC
Abstract
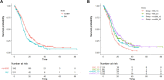
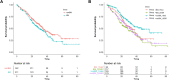
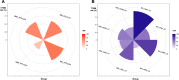
Non-small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) mutation is brain metastasis (BM)-prone. We determined the impact of this hallmark, along with EGFR subtype and generation of tyrosine kinase inhibitor (TKI) treatment, on patients' outcome. 553 metastatic EGFR-mutant NSCLC patients received front-line EGFR-TKI treatment. Progression-free survival (PFS), overall survival (OS) and secondary T790M rate were analysed. BM was observed in 211 (38.2%) patients. BM (HR 1.20 [95% CI 0.99-1.48]; p = 0.053), ECOG PS 0-1 (HR 0.71 [95% CI 0.54-0.93]; p = 0.014) and afatinib treatment (HR 0.81 [95% CI 0.66-0.99]; p = 0.045) were associated with PFS. Afatinib-treated patients without BM demonstrated a significantly longer PFS (16.3 months) compared to afatinib-treated patients with BM (13.7 months) and to gefitinib/erlotinib-treated patients with (11.1 months) or without BM (14.2 months; p < 0.001). CNS-only progression trended higher in afatinib-treated patients. ECOG PS 0-1 (HR 0.41 [95% CI 0.31-0.56]; p < 0.001) and EGFR L858R mutation (HR 1.46 [95% CI 1.13-1.88]; p = 0.003), but not BM, were the predictors for OS. BM (OR 2.02 [95% CI 1.02-4.08]; p = 0.040), afatinib treatment (OR 0.26 [95% CI 0.12-0.50]; p < 0.001) and EGFR L858R mutation (OR 0.55 [95% CI 0.28-1.05]; p = 0.070) were associated with secondary T790M rate. In BM patients, gefitinib/erlotinib-treated ones with 19 deletion mutation and afatinib-treated ones with L858R mutation had the highest and the lowest T790M rate (94.4% vs. 27.3%, p < 0.001), respectively. BM and generation of EGFR-TKI jointly impact PFS and secondary T790M rate in patients with EGFR-mutant NSCLC, whereas OS was mainly associated with EGFR subtype.
© 2023. The Author(s).
Conflict of interest statement
CS-K received speaker honoraria from AstraZeneca, Boehringer Ingelheim, Roche, Pfizer, Eli Lilliy, Novartis, OnO Pharma, Chugai, Merck, Janssen Pharma, Takeda and Guardant Health. CS-K provided consultation for AstraZeneca, Boehringer Ingelheim, Eli Lilliy, Merck, Chugai, Takeda, Novartis and Guardant Health. None of the other authors have any conflict of interest to disclose.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

