Loading of green-synthesized cu nanoparticles on Ag complex containing 1,3,5-triazine Schiff base with enhanced antimicrobial activities
- PMID: 37989862
- PMCID: PMC10663565
- DOI: 10.1038/s41598-023-47358-4
Loading of green-synthesized cu nanoparticles on Ag complex containing 1,3,5-triazine Schiff base with enhanced antimicrobial activities
Abstract
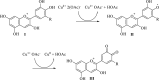
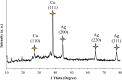
The physicochemical properties of materials change significantly in nanometer dimensions. Therefore, several methods have been proposed for the synthesis of nanoparticles. Plant extracts and essential oils are applied as natural and economic resources to prepare nanomaterials especially metal nanoparticles. In this project, a green, simple and efficient method has been designed for the synthesis of Cu nanoparticles using Purple cabbage extract as a reducing and stabilizing agent. They were successfully loaded onto a new Ag complex containing 1,3,5-triazine Schiff base as ligand to form Cu@Ag-CPX nanocomposite. Phytochemical contents of extract were identified by standard qualitative analyses. The chemical structure of all synthesized compounds was characterized using spectral data. In FT-IR, coordination of C=N bond of Schiff base ligand to Ag+ ions shifted the absorption band from 1641 to 1632 cm-1. The UV-Vis spectrum of Cu@Ag-CPX nanocomposite shown the peak related to Cu nanoparticles in the region of around 251 nm. 5:7 molar ratio of Cu to Ag in Cu@Ag-CPX was determined using ICP-OES. The FESEM, TEM, and DLS techniques provided valuable insights into the morphology and size distribution of the nanocomposite, revealing the presence of rods and monodispersed particles with specific diameter ranges. These analyses of the nanocomposite displayed rods with diameters from 40 to 62 nm as well as monodispersed and uniform particles with average diameter of 45 nm, respectively. The presence of elements including carbon, nitrogen, oxygen, Cu and Ag was proved by EDX-EDS analysis. The XRD pattern of Cu@Ag-CPX shown the diffraction peaks of Cu and Ag particles at 2θ values of 10°-80°, and confirmed its crystalline nature. The inhibitory properties of the synthesized compounds were evaluated in vitro against four Gram-negative and two Gram-positive bacteria, as well as two fungal strains. The MIC, MBC and MFC values obtained from microdilution and streak plate sensitivity tests were ranged from 128 to 4096 µg ml-1. While Cu nanoparticles and Ag complexes were effective against some pathogens, they were not effective against all them. However, the growth of all tested microbial strains was inhibited by Cu@Ag-CPX nanocomposite, and makes it as a new promising antimicrobial agent. Modification of nanocomposite in terms of nanoparticle and complex can improve its blocking activities.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests
Figures







Similar articles
-
Characterization and Evaluation of Antimicrobial Potential of Trigonella incise (Linn) Mediated Biosynthesized Silver Nanoparticles.Molecules. 2022 Jul 20;27(14):4618. doi: 10.3390/molecules27144618. Molecules. 2022. PMID: 35889490 Free PMC article.
-
Study on antibacterial alginate-stabilized copper nanoparticles by FT-IR and 2D-IR correlation spectroscopy.Int J Nanomedicine. 2012;7:3597-612. doi: 10.2147/IJN.S32648. Epub 2012 Jul 11. Int J Nanomedicine. 2012. PMID: 22848180 Free PMC article.
-
Green synthesis of Ag and Cu-doped Bismuth oxide nanoparticles: Revealing synergistic antimicrobial and selective cytotoxic potentials for biomedical advancements.J Trace Elem Med Biol. 2024 Jan;81:127325. doi: 10.1016/j.jtemb.2023.127325. Epub 2023 Oct 20. J Trace Elem Med Biol. 2024. PMID: 37922658
-
Green synthesis, characterization of silver nanoparticals for biomedical application and environmental remediation.J Microbiol Methods. 2022 Feb;193:106384. doi: 10.1016/j.mimet.2021.106384. Epub 2021 Nov 23. J Microbiol Methods. 2022. PMID: 34826520 Review.
-
Antimicrobial Properties of the Ag, Cu Nanoparticle System.Biology (Basel). 2021 Feb 10;10(2):137. doi: 10.3390/biology10020137. Biology (Basel). 2021. PMID: 33578705 Free PMC article. Review.
Cited by
-
Fine-Tuned Graphene Oxide Nanocomposite: Harnessing Copper(II)-Imidazole Complex for Enhanced Biological Responses and Balanced Photocatalytic Functionality.Materials (Basel). 2024 Feb 15;17(4):892. doi: 10.3390/ma17040892. Materials (Basel). 2024. PMID: 38399142 Free PMC article.
-
Unexpected stability of the iron(II) complex by an asymmetrical Schiff base from Fe(III): structure, magnetic and Mössbauer investigations.R Soc Open Sci. 2025 Jan 8;12(1):241334. doi: 10.1098/rsos.241334. eCollection 2025 Jan. R Soc Open Sci. 2025. PMID: 39780966 Free PMC article.
-
Green synthesis of silver nanoparticles from plant Astragalus fasciculifolius Bioss and evaluating cytotoxic effects on MCF7 human breast cancer cells.Sci Rep. 2025 Jul 15;15(1):25474. doi: 10.1038/s41598-025-05224-5. Sci Rep. 2025. PMID: 40664749 Free PMC article.
References
-
- Yang X, et al. A new method for conversion of fructose and glucose to 5-hydroxymethylfurfural by magnetic mesoporous of SBA-16 was modified to sulfonic acid as Lewis’s acid catalysts. Renew. Energy. 2023;209:145–156. doi: 10.1016/j.renene.2023.03.102. - DOI
-
- Niakan M, Masteri-Farahani M, Karimi S, Shekaari H. Sulfonic acid functionalized dendrimer-grafted cellulose as a solid acid catalyst for the high-yield and green production of 5-hydroxymethylfurfural. Sustain. Energy Fuels. 2022;6:2514–2522. doi: 10.1039/D2SE00235C. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

