Mutation at the entrance of the quinone cavity severely disrupts quinone binding in respiratory complex I
- PMID: 37989876
- PMCID: PMC10663621
- DOI: 10.1038/s41598-023-47314-2
Mutation at the entrance of the quinone cavity severely disrupts quinone binding in respiratory complex I
Abstract
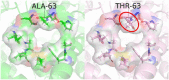
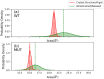
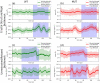
In all resolved structures of complex I, there exists a tunnel-like Q-chamber for ubiquinone binding and reduction. The entrance to the Q-chamber in ND1 subunit forms a narrow bottleneck, which is rather tight and requires thermal conformational changes for ubiquinone to get in and out of the binding chamber. The substitution of alanine with threonine at the bottleneck (AlaThr MUT), associated with 3460/ND1 mtDNA mutation in human complex I, is implicated in Leber's Hereditary Optic Neuropathy (LHON). Here, we show the AlaThr MUT further narrows the Q-chamber entrance cross-section area by almost 30%, increasing the activation free energy barrier of quinone passage by approximately 5 kJ mol-1. This severely disrupts quinone binding and reduction as quinone passage through the bottleneck is slowed down almost tenfold. Our estimate of the increase in free energy barrier is entirely due to the bottleneck narrowing, leading to a reduction of the transition state entropy between WT and MUT, and thus more difficult quinone passage. Additionally, we investigate details of possible water exchange between the Q-chamber and membrane. We find water exchange is dynamic in WT but may be severely slowed in MUT. We propose that LHON symptoms caused by 3460/ND1 mtDNA mutation are due to slowed quinone binding. This leads to an increased production of reactive oxidative species due to upstream electron backup at the FMN site of complex I, thus resulting in a mt bioenergetic defect.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
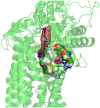


Similar articles
-
Quinone binding in respiratory complex I: Going through the eye of a needle. The squeeze-in mechanism of passing the narrow entrance of the quinone site.Photochem Photobiol Sci. 2022 Jan;21(1):1-12. doi: 10.1007/s43630-021-00113-y. Epub 2021 Nov 23. Photochem Photobiol Sci. 2022. PMID: 34813075 Free PMC article.
-
Leber hereditary optic neuropathy mutations in the ND6 subunit of mitochondrial complex I affect ubiquinone reduction kinetics in a bacterial model of the enzyme.Biochem J. 2008 Jan 1;409(1):129-37. doi: 10.1042/BJ20070866. Biochem J. 2008. PMID: 17894548
-
LHON/MELAS overlap mutation in ND1 subunit of mitochondrial complex I affects ubiquinone binding as revealed by modeling in Escherichia coli NDH-1.Biochim Biophys Acta. 2012 Feb;1817(2):312-8. doi: 10.1016/j.bbabio.2011.10.014. Epub 2011 Nov 4. Biochim Biophys Acta. 2012. PMID: 22079202
-
Leber's Hereditary Optic Neuropathy as a Promising Disease for Gene Therapy Development.Adv Ther. 2019 Dec;36(12):3299-3307. doi: 10.1007/s12325-019-01113-2. Epub 2019 Oct 11. Adv Ther. 2019. PMID: 31605306 Free PMC article.
-
Leber's hereditary optic neuropathy: a multifactorial disease.Prog Retin Eye Res. 2006 Jul;25(4):381-96. doi: 10.1016/j.preteyeres.2006.05.002. Epub 2006 Jul 7. Prog Retin Eye Res. 2006. PMID: 16829155 Review.
Cited by
-
Searching for proton transfer channels in respiratory complex I.Biophys J. 2024 Dec 17;123(24):4233-4244. doi: 10.1016/j.bpj.2024.07.041. Epub 2024 Aug 7. Biophys J. 2024. PMID: 39095988 Free PMC article.
-
A systematic review on the biochemical threshold of mitochondrial genetic variants.Genome Res. 2024 Apr 25;34(3):341-365. doi: 10.1101/gr.278200.123. Genome Res. 2024. PMID: 38627095 Free PMC article.

