Intestinal Atp8b1 dysfunction causes hepatic choline deficiency and steatohepatitis
- PMID: 37990006
- PMCID: PMC10663612
- DOI: 10.1038/s41467-023-42424-x
Intestinal Atp8b1 dysfunction causes hepatic choline deficiency and steatohepatitis
Abstract
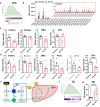
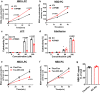
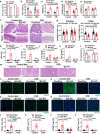
Choline is an essential nutrient, and its deficiency causes steatohepatitis. Dietary phosphatidylcholine (PC) is digested into lysoPC (LPC), glycerophosphocholine, and choline in the intestinal lumen and is the primary source of systemic choline. However, the major PC metabolites absorbed in the intestinal tract remain unidentified. ATP8B1 is a P4-ATPase phospholipid flippase expressed in the apical membrane of the epithelium. Here, we use intestinal epithelial cell (IEC)-specific Atp8b1-knockout (Atp8b1IEC-KO) mice. These mice progress to steatohepatitis by 4 weeks. Metabolomic analysis and cell-based assays show that loss of Atp8b1 in IEC causes LPC malabsorption and thereby hepatic choline deficiency. Feeding choline-supplemented diets to lactating mice achieves complete recovery from steatohepatitis in Atp8b1IEC-KO mice. Analysis of samples from pediatric patients with ATP8B1 deficiency suggests its translational potential. This study indicates that Atp8b1 regulates hepatic choline levels through intestinal LPC absorption, encouraging the evaluation of choline supplementation therapy for steatohepatitis caused by ATP8B1 dysfunction.
© 2023. The Author(s).
Conflict of interest statement
H.H., Y.S. and R.T. are coinventors on a patent (PCT/JP2022/44650) on therapy with choline metabolites for diseases caused by ATP8B1 dysfunction. The other authors declare no competing interests.
Figures




References
-
- Leermakers ETM, et al. Effects of choline on health across the life course: a systematic review. Nutr. Rev. 2015;73:500–522. - PubMed
-
- Yao ZM, Vance DE. Reduction in VLDL, but not HDL, in plasma of rats deficient in choline. Biochem. Cell Biol. 1990;68:552–558. - PubMed
-
- Yao ZM, Vance DE. The active synthesis of phosphatidylcholine is required for very low density lipoprotein secretion from rat hepatocytes. J. Biol. Chem. 1988;263:2998–3004. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

