Hepatocyte integrity depends on c-Jun-controlled proliferation in Schistosoma mansoni infected mice
- PMID: 37990129
- PMCID: PMC10663609
- DOI: 10.1038/s41598-023-47646-z
Hepatocyte integrity depends on c-Jun-controlled proliferation in Schistosoma mansoni infected mice
Abstract
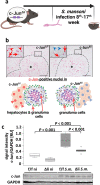
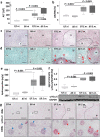
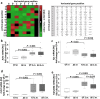
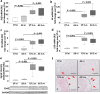
Schistosomiasis is a parasitic disease affecting more than 250 million people worldwide. The transcription factor c-Jun, which is induced in S. mansoni infection-associated liver disease, can promote hepatocyte survival but can also trigger hepatocellular carcinogenesis. We aimed to analyze the hepatic role of c-Jun following S. mansoni infection. We adopted a hepatocyte-specific c-Jun knockout mouse model (Alb-Cre/c-Jun loxP) and analyzed liver tissue and serum samples by quantitative real-time PCR array, western blotting, immunohistochemistry, hydroxyproline quantification, and functional analyses. Hepatocyte-specific c-Jun knockout (c-JunΔli) was confirmed by immunohistochemistry and western blotting. Infection with S. mansoni induced elevated aminotransferase-serum levels in c-JunΔli mice. Of note, hepatic Cyclin D1 expression was induced in infected c-Junf/f control mice but to a lower extent in c-JunΔli mice. S. mansoni soluble egg antigen-induced proliferation in a human hepatoma cell line was diminished by inhibition of c-Jun signaling. Markers for apoptosis, oxidative stress, ER stress, inflammation, autophagy, DNA-damage, and fibrosis were not altered in S. mansoni infected c-JunΔli mice compared to infected c-Junf/f controls. Enhanced liver damage in c-JunΔli mice suggested a protective role of c-Jun. A reduced Cyclin D1 expression and reduced hepatic regeneration could be the reason. In addition, it seems likely that the trends in pathological changes in c-JunΔli mice cumulatively led to a loss of the protective potential being responsible for the increased hepatocyte damage and loss of regenerative ability.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- WHO. Schistosomiasis Fact Sheet, 02_2023; Available from: https://www.who.int/news-room/fact-sheets/detail/schistosomiasis.
-
- Salas-Coronas J, Bargues MD, Lozano-Serrano AB, Artigas P, Martínez-Ortí A, Mas-Coma S, et al. Evidence of autochthonous transmission of urinary schistosomiasis in Almeria (southeast Spain): An outbreak analysis. Travel Med. Infect. Dis. 2021;44:102165. doi: 10.1016/j.tmaid.2021.102165. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

