Circular RNA ZBTB46 depletion alleviates the progression of Atherosclerosis by regulating the ubiquitination and degradation of hnRNPA2B1 via the AKT/mTOR pathway
- PMID: 37990246
- PMCID: PMC10662463
- DOI: 10.1186/s12979-023-00386-0
Circular RNA ZBTB46 depletion alleviates the progression of Atherosclerosis by regulating the ubiquitination and degradation of hnRNPA2B1 via the AKT/mTOR pathway
Abstract
Background: CircZBTB46 has been identified as being associated with the risk of coronary artery disease (CAD) and has the potential to be a diagnostic biomarker for CAD. However, the specific function and detailed mechanism of circZBTB46 in CAD are still unknown.
Methods: The expression levels and properties of circRNAs were examined using qRT‒PCR, RNA FISH, and subcellular localization analysis. ApoE-/- mice fed a high-fat diet were used to establish an atherosclerosis model. HE, Masson, and Oil Red O staining were used to analyze the morphological features of the plaque. CCK-8, Transwell, and wound healing assays, and flow cytometric analysis were used to evaluate cell proliferation, migration, and apoptosis. RNA pull-down, silver staining, mass spectrometry analysis, and RNA-binding protein immunoprecipitation (RIP) were performed to identify the interacting proteins of circZBTB46.
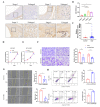
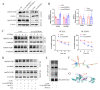
Results: CircZBTB46 is highly conserved and is significantly upregulated in atherosclerotic lesions. Functional studies revealed that knockdown of circZBTB46 significantly decreased the atherosclerotic plaque area, attenuating the progression of atherosclerosis. In addition, silencing circZBTB46 inhibited cell proliferation and migration and induced apoptosis. Mechanistically, circZBTB46 physically interacted with hnRNPA2B1 and suppressed its degradation, thereby regulating cell functions and the formation of aortic atherosclerotic plaques. Additionally, circZBTB46 was identified as a functional mediator of PTEN-dependent regulation of the AKT/mTOR signaling pathway and thus affected cell proliferation and migration and induced apoptosis.
Conclusion: Our study provides the first direct evidence that circZBTB46 functions as an important regulatory molecule for CAD progression by interacting with hnRNPA2B1 and regulating the PTEN/AKT/mTOR pathway.
Keywords: Atherosclerotic plaque; circular RNA; Coronary artery Disease; RNA-binding protein.
© 2023. The Author(s).
Conflict of interest statement
There are no conflicts of interest associated with this manuscript.
Figures




Similar articles
-
CircZBTB46 Protects Acute Myeloid Leukemia Cells from Ferroptotic Cell Death by Upregulating SCD.Cancers (Basel). 2023 Jan 11;15(2):459. doi: 10.3390/cancers15020459. Cancers (Basel). 2023. PMID: 36672408 Free PMC article.
-
Down-regulation of the Smad signaling by circZBTB46 via the Smad2-PDLIM5 axis to inhibit type I collagen expression.J Geriatr Cardiol. 2023 Jun 28;20(6):431-447. doi: 10.26599/1671-5411.2023.06.002. J Geriatr Cardiol. 2023. PMID: 37416515 Free PMC article.
-
NUF2 promotes tumorigenesis by interacting with HNRNPA2B1 via PI3K/AKT/mTOR pathway in ovarian cancer.J Ovarian Res. 2023 Jan 20;16(1):17. doi: 10.1186/s13048-023-01101-9. J Ovarian Res. 2023. PMID: 36670423 Free PMC article.
-
Nuciferine attenuates atherosclerosis by regulating the proliferation and migration of VSMCs through the Calm4/MMP12/AKT pathway in ApoE(-/-) mice fed with High-Fat-Diet.Phytomedicine. 2023 Jan;108:154536. doi: 10.1016/j.phymed.2022.154536. Epub 2022 Nov 9. Phytomedicine. 2023. PMID: 36395561
-
Detailed profiling of m6A modified circRNAs and synergistic effects of circRNA and environmental risk factors for coronary artery disease.Eur J Pharmacol. 2023 Jul 15;951:175761. doi: 10.1016/j.ejphar.2023.175761. Epub 2023 May 9. Eur J Pharmacol. 2023. PMID: 37169142
Cited by
-
HNRNPA2B1: a novel target in pulmonary arterial hypertension.Front Cardiovasc Med. 2025 Jul 9;12:1497938. doi: 10.3389/fcvm.2025.1497938. eCollection 2025. Front Cardiovasc Med. 2025. PMID: 40703632 Free PMC article. Review.
-
The Dark Side of Vascular Aging: Noncoding Ribonucleic Acids in Heart Failure with Preserved Ejection Fraction.Cells. 2025 Aug 16;14(16):1269. doi: 10.3390/cells14161269. Cells. 2025. PMID: 40862748 Free PMC article. Review.
-
CircRNA Networks in CAD: Multi-Cellular Mechanisms and Clinical Potential.Int J Gen Med. 2025 Jun 12;18:3129-3150. doi: 10.2147/IJGM.S524189. eCollection 2025. Int J Gen Med. 2025. PMID: 40529348 Free PMC article. Review.
-
Circular RNA in Cardiovascular Diseases: Biogenesis, Function and Application.Biomolecules. 2024 Aug 6;14(8):952. doi: 10.3390/biom14080952. Biomolecules. 2024. PMID: 39199340 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

