Anti-Obesity Medications and Investigational Agents: An Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) 2022
- PMID: 37990711
- PMCID: PMC10662004
- DOI: 10.1016/j.obpill.2022.100018
Anti-Obesity Medications and Investigational Agents: An Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) 2022
Abstract
Background: This "Anti-Obesity Medications and Investigational Agents: An Obesity Medicine Association Clinical Practice Statement 2022" is intended to provide clinicians an overview of Food and Drug Administration (FDA) approved anti-obesity medications and investigational anti-obesity agents in development.
Methods: The scientific information for this Clinical Practice Statement (CPS) is based upon published scientific citations, clinical perspectives of OMA authors, and peer review by the Obesity Medicine Association leadership.
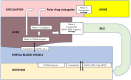
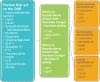
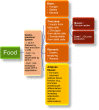
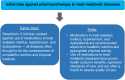
Results: This CPS describes pharmacokinetic principles applicable to those with obesity, and discusses the efficacy and safety of anti-obesity medications [e.g., phentermine, semaglutide, liraglutide, phentermine/topiramate, naltrexone/bupropion, and orlistat, as well as non-systemic superabsorbent oral hydrogel particles (which is technically classified as a medical device)]. Other medications discussed include setmelanotide, metreleptin, and lisdexamfetamine dimesylate. Data regarding the use of combination anti-obesity pharmacotherapy, as well as use of anti-obesity pharmacotherapy after bariatric surgery are limited; however, published data support such approaches. Finally, this CPS discusses investigational anti-obesity medications, with an emphasis on the mechanisms of action and summary of available clinical trial data regarding tirzepatide.
Conclusion: This "Anti-Obesity Medications and Investigational Agents: An Obesity Medicine Association Clinical Practice Statement 2022" is one of a series of OMA CPSs designed to assist clinicians in the care of patients with pre-obesity/obesity.
Keywords: Anti-obesity medications; Investigational agents; Obesity; Semaglutide; Tirzepatide.
© 2022 The Authors.
Figures




Similar articles
-
Thirty Obesity Myths, Misunderstandings, and/or Oversimplifications: An Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) 2022.Obes Pillars. 2022 Aug 10;3:100034. doi: 10.1016/j.obpill.2022.100034. eCollection 2022 Sep. Obes Pillars. 2022. PMID: 37990730 Free PMC article.
-
Stress, psychiatric disease, and obesity: An Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) 2022.Obes Pillars. 2022 Nov 4;4:100041. doi: 10.1016/j.obpill.2022.100041. eCollection 2022 Dec. Obes Pillars. 2022. PMID: 37990662 Free PMC article.
-
Bariatric surgery, gastrointestinal hormones, and the microbiome: An Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) 2022.Obes Pillars. 2022 Apr 1;2:100015. doi: 10.1016/j.obpill.2022.100015. eCollection 2022 Jun. Obes Pillars. 2022. PMID: 37990718 Free PMC article.
-
Long-term effects of weight-reducing drugs in people with hypertension.Cochrane Database Syst Rev. 2021 Jan 17;1(1):CD007654. doi: 10.1002/14651858.CD007654.pub5. Cochrane Database Syst Rev. 2021. PMID: 33454957 Free PMC article.
-
Long-Term Efficacy and Safety of Anti-Obesity Treatment: Where Do We Stand?Curr Obes Rep. 2021 Mar;10(1):14-30. doi: 10.1007/s13679-020-00422-w. Epub 2021 Jan 6. Curr Obes Rep. 2021. PMID: 33410104 Free PMC article. Review.
Cited by
-
Obesity and hypertension: Obesity medicine association (OMA) clinical practice statement (CPS) 2023.Obes Pillars. 2023 Aug 7;8:100083. doi: 10.1016/j.obpill.2023.100083. eCollection 2023 Dec. Obes Pillars. 2023. PMID: 38125655 Free PMC article.
-
Obesity, dyslipidemia, and cardiovascular disease: A joint expert review from the Obesity Medicine Association and the National Lipid Association 2024.Obes Pillars. 2024 Mar 12;10:100108. doi: 10.1016/j.obpill.2024.100108. eCollection 2024 Jun. Obes Pillars. 2024. PMID: 38706496 Free PMC article.
-
Weight loss efficiency and safety of tirzepatide: A Systematic review.PLoS One. 2023 May 4;18(5):e0285197. doi: 10.1371/journal.pone.0285197. eCollection 2023. PLoS One. 2023. PMID: 37141329 Free PMC article.
-
Melanocortin 4 receptor mutation in obesity.World J Exp Med. 2024 Dec 20;14(4):99239. doi: 10.5493/wjem.v14.i4.99239. eCollection 2024 Dec 20. World J Exp Med. 2024. PMID: 39713072 Free PMC article. Review.
-
Obesity, thrombosis, venous disease, lymphatic disease, and lipedema: An obesity medicine association (OMA) clinical practice statement (CPS) 2023.Obes Pillars. 2023 Oct 19;8:100092. doi: 10.1016/j.obpill.2023.100092. eCollection 2023 Dec. Obes Pillars. 2023. PMID: 38125656 Free PMC article.
References
-
- Bays H.E., McCarthy W., Burridge K., Tondt J., Karjoo S., Christensen S., Ng J., Golden A., Davisson L., Richardson L. Obesity algorithm eBook. 2021. www.obesityalgorithm.orghttps://obesitymedicine.org/obesity-algorithm/ presented by the Obesity Medicine Association.
-
- Bray G.A. Why do we need drugs to treat the patient with obesity? Obesity. 2013;21:893–899. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

