Quisinostat is a brain-penetrant radiosensitizer in glioblastoma
- PMID: 37991020
- PMCID: PMC10721329
- DOI: 10.1172/jci.insight.167081
Quisinostat is a brain-penetrant radiosensitizer in glioblastoma
Abstract
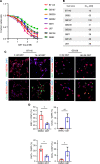
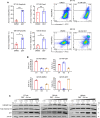
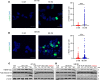
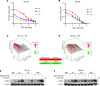
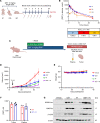
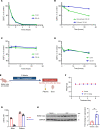
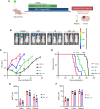
Histone deacetylase (HDAC) inhibitors have garnered considerable interest for the treatment of adult and pediatric malignant brain tumors. However, owing to their broad-spectrum nature and inability to effectively penetrate the blood-brain barrier, HDAC inhibitors have failed to provide substantial clinical benefit to patients with glioblastoma (GBM) to date. Moreover, global inhibition of HDACs results in widespread toxicity, highlighting the need for selective isoform targeting. Although no isoform-specific HDAC inhibitors are currently available, the second-generation hydroxamic acid-based HDAC inhibitor quisinostat possesses subnanomolar specificity for class I HDAC isoforms, particularly HDAC1 and HDAC2. It has been shown that HDAC1 is the essential HDAC in GBM. This study analyzed the neuropharmacokinetic, pharmacodynamic, and radiation-sensitizing properties of quisinostat in preclinical models of GBM. It was found that quisinostat is a well-tolerated and brain-penetrant molecule that extended survival when administered in combination with radiation in vivo. The pharmacokinetic-pharmacodynamic-efficacy relationship was established by correlating free drug concentrations and evidence of target modulation in the brain with survival benefit. Together, these data provide a strong rationale for clinical development of quisinostat as a radiosensitizer for the treatment of GBM.
Keywords: Brain cancer; Drug therapy; Oncology; Radiation therapy; Therapeutics.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

