Anti-NF-κB peptide derived from nuclear acidic protein attenuates ovariectomy-induced osteoporosis in mice
- PMID: 37991021
- PMCID: PMC10721323
- DOI: 10.1172/jci.insight.171962
Anti-NF-κB peptide derived from nuclear acidic protein attenuates ovariectomy-induced osteoporosis in mice
Abstract
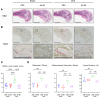
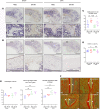
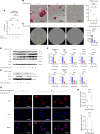
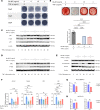
NF-κB is a transcription factor that is activated with aging. It plays a key role in the development of osteoporosis by promoting osteoclast differentiation and inhibiting osteoblast differentiation. In this study, we developed a small anti-NF-κB peptide called 6A-8R from a nuclear acidic protein (also known as macromolecular translocation inhibitor II, Zn2+-binding protein, or parathymosin) that inhibits transcriptional activity of NF-κB without altering its nuclear translocation and binding to DNA. Intraperitoneal injection of 6A-8R attenuated ovariectomy-induced osteoporosis in mice by inhibiting osteoclast differentiation, promoting osteoblast differentiation, and inhibiting sclerostin production by osteocytes in vivo with no apparent side effects. Conversely, in vitro, 6A-8R inhibited osteoclast differentiation by inhibiting NF-κB transcriptional activity, promoted osteoblast differentiation by promoting Smad1 phosphorylation, and inhibited sclerostin expression in osteocytes by inhibiting myocyte enhancer factors 2C and 2D. These findings suggest that 6A-8R has the potential to be an antiosteoporotic therapeutic agent with uncoupling properties.
Keywords: Bone Biology; Mouse models; NF-kappaB; Osteoporosis.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

