Biomimetic Macrophage Membrane-Camouflaged Nanoparticles Induce Ferroptosis by Promoting Mitochondrial Damage in Glioblastoma
- PMID: 37991252
- PMCID: PMC10722604
- DOI: 10.1021/acsnano.3c07555
Biomimetic Macrophage Membrane-Camouflaged Nanoparticles Induce Ferroptosis by Promoting Mitochondrial Damage in Glioblastoma
Abstract
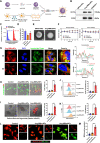
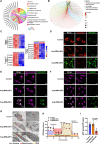
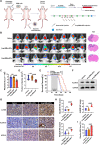
The increasing understanding of ferroptosis has indicated its role and therapeutic potential in cancer; however, this knowledge has yet to be translated into effective therapies. Glioblastoma (GBM) patients face a bleak prognosis and encounter challenges due to the limited treatment options available. In this study, we conducted a genome-wide CRISPR-Cas9 screening in the presence of a ferroptosis inducer (RSL3) to identify the key driver genes involved in ferroptosis. We identified ALOX15, a key lipoxygenase (LOX), as an essential driver of ferroptosis. Small activating RNA (saRNA) was used to mediate the expression of ALOX15 promoted ferroptosis in GBM cells. We then coated saALOX15-loaded mesoporous polydopamine (MPDA) with Angiopep-2-modified macrophage membranes (MMs) to reduce the clearance by the mononuclear phagocyte system (MPS) and increase the ability of the complex to cross the blood-brain barrier (BBB) during specific targeted therapy of orthotopic GBM. These generated hybrid nanoparticles (NPs) induced ferroptosis by mediating mitochondrial dysfunction and rendering mitochondrial morphology abnormal. In vivo, the modified MM enabled the NPs to target GBM cells, exert a marked inhibitory effect on GBM progression, and promote GBM radiosensitivity. Our results reveal ALOX15 to be a promising therapeutic target in GBM and suggest a biomimetic strategy that depends on the biological properties of MMs to enhance the in vivo performance of NPs for treating GBM.
Keywords: ALOX15; ferroptosis; glioblastoma; macrophage membrane; nanoparticle.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Gu J.; Mu N.; Jia B.; Guo Q.; Pan L.; Zhu M.; Zhang W.; Zhang K.; Li W.; Li M.; Wei L.; Xue X.; Zhang Y.; Zhang W. Targeting radiation-tolerant persister cells as a strategy for inhibiting radioresistance and recurrence in glioblastoma. Neuro Oncol 2022, 24 (7), 1056–1070. 10.1093/neuonc/noab288. - DOI - PMC - PubMed
-
- Liu T.; Zhu C.; Chen X.; Guan G.; Zou C.; Shen S.; Wu J.; Wang Y.; Lin Z.; Chen L.; Cheng P.; Cheng W.; Wu A. Ferroptosis, as the most enriched programmed cell death process in glioma, induces immunosuppression and immunotherapy resistance. Neuro Oncol 2022, 24 (7), 1113–1125. 10.1093/neuonc/noac033. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

