Comparative Treatment Persistence and Adherence to Endothelin Receptor Antagonists Among Patients with Pulmonary Arterial Hypertension in Japan: A Real-World Administrative Claims Database Study
- PMID: 37991630
- PMCID: PMC10721767
- DOI: 10.1007/s41030-023-00244-w
Comparative Treatment Persistence and Adherence to Endothelin Receptor Antagonists Among Patients with Pulmonary Arterial Hypertension in Japan: A Real-World Administrative Claims Database Study
Abstract
Introduction: Real-world data on the comparative effectiveness of endothelin receptor antagonists (ERAs; macitentan, bosentan, ambrisentan) for pulmonary arterial hypertension (PAH), particularly in Asian countries, are scarce. We evaluated the persistence of these ERAs before and after macitentan approval in Japan (2015).
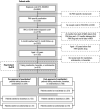
Methods: We used real-world data from the Japanese Medical Data Vision administrative claims database between April 2008 and November 2020. Patients with PAH were identified from the dataset. Persistence to ERA treatment before and after approval of macitentan in Japan was defined as the time between start of the index ERA and treatment discontinuation or death. Propensity score adjustment was applied to minimize confounding effects among treatment groups.
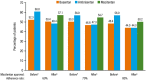
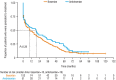
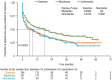
Results: In the pre-macitentan approval cohort, 153 and 51 patients received bosentan and ambrisentan, respectively. In the post-macitentan approval cohort, 331, 284, and 91 patients received macitentan, bosentan, and ambrisentan, respectively. Unadjusted median persistence for ambrisentan- and bosentan-treated patients was 19 and 10 months, respectively (adjusted HR 0.87 [95% CI 0.61-1.24]; P = 0.434 [bosentan as reference]). In the post-macitentan approval cohort, unadjusted median persistence was 18 months for macitentan-treated patients versus 6 and 8 months for ambrisentan- and bosentan-treated patients, respectively. Adjusted HRs for ambrisentan and bosentan were 1.48 (95% CI 1.12-1.95; P = 0.006) and 1.63 (95% CI 1.30-2.04; P < 0.001 [macitentan as reference]), respectively.
Conclusions: Real-world data for Japanese patients with PAH showed that persistence was significantly higher for macitentan, versus ambrisentan and bosentan, since its approval.
Keywords: Endothelin receptor antagonists; Japanese patients; Pulmonary arterial hypertension; Real-world data; Treatment persistence.
© 2023. The Author(s).
Conflict of interest statement
Junichi Omura is an employee of Janssen Pharmaceuticals. Yogeshwar Makanji is an employee of Janssen Pharmaceuticals. Nobuhiro Tanabe has nothing to disclose. Dae Young Yu is an employee of Janssen Pharmaceuticals. Jin Yu Tan is an employee of Janssen Pharmaceuticals. Sooyeol Lim is a former employee of Prospection, which received funding from Janssen to conduct the study analyses. Mahsa H. Kouhkamari is a former employee of Prospection, which received funding from Janssen to conduct the study analyses. Jeremy Casorso is a current employee of Prospection, which received funding from Janssen to conduct the study analyses. David Bin-Chia Wu is an employee of Janssen Pharmaceuticals. Paul Bloomfield was an employee of Janssen Pharmaceuticals at the time of the analysis.
Figures
References
LinkOut - more resources
Full Text Sources
Miscellaneous

