Optimized, automated and cGMP-compliant synthesis of the HER2 targeting [68Ga]Ga-ABY-025 tracer
- PMID: 37991639
- PMCID: PMC10665286
- DOI: 10.1186/s41181-023-00226-y
Optimized, automated and cGMP-compliant synthesis of the HER2 targeting [68Ga]Ga-ABY-025 tracer
Abstract
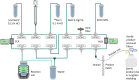
Background: The Affibody molecule, ABY-025, has demonstrated utility to detect human epidermal growth factor receptor 2 (HER2) in vivo, either radiolabelled with indium-111 (111In) or gallium-68 (68Ga). Using the latter, 68Ga, is preferred due to its use in positron emission tomography with superior resolution and quantifying capabilities in the clinical setting compared to 111In. For an ongoing phase II study (NCT05619016) evaluating ABY-025 for detecting HER2-low lesions and selection of patients for HER2-targeted treatment, the aim was to optimize an automated and cGMP-compliant radiosynthesis of [68Ga]Ga-ABY-025. [68Ga]Ga-ABY-025 was produced on a synthesis module, Modular-Lab PharmTracer (Eckert & Ziegler), commonly used for 68Ga-labelings. The radiotracer has previously been radiolabeled on this module, but to streamline the production, the method was optimized. Steps requiring manual interactions to the radiolabeling procedure were minimized including a convenient and automated pre-concentration of the 68Ga-eluate and a simplified automated final formulation procedure. Every part of the radiopharmaceutical production was carefully developed to gain robustness and to avoid any operator bound variations to the manufacturing. The optimized production method was successfully applied for 68Ga-labeling of another radiotracer, verifying its versatility as a universal and robust method for radiosynthesis of Affibody-based peptides.
Results: A simplified and optimized automated cGMP-compliant radiosynthesis method of [68Ga]Ga-ABY-025 was developed. With a decay corrected radiochemical yield of 44 ± 2%, a radiochemical purity (RCP) of 98 ± 1%, and with an RCP stability of 98 ± 1% at 2 h after production, the method was found highly reproducible. The production method also showed comparable results when implemented for radiolabeling another similar peptide.
Conclusion: The improvements made for the radiosynthesis of [68Ga]Ga-ABY-025, including introducing a pre-concentration of the 68Ga-eluate, aimed to utilize the full potential of the 68Ge/68Ga generator radioactivity output, thereby reducing radioactivity wastage. Furthermore, reducing the number of manually performed preparative steps prior to the radiosynthesis, not only minimized the risk of potential human/operator errors but also enhanced the process' robustness. The successful application of this optimized radiosynthesis method to another similar peptide underscores its versatility, suggesting that our method can be adopted for 68Ga-labeling radiotracers based on Affibody molecules in general.
Trial registration: NCT, NCT05619016, Registered 7 November 2022, https://clinicaltrials.gov/study/NCT05619016?term=HER2&cond=ABY025&rank=1.
Keywords: ABY-025; Affibody; Breast cancer; Gallium-68; HER2 low; HER2-PET imaging; Human Epidermal growth factor Receptor 2; [68Ga]Ga-ABY-025.
© 2023. The Author(s).
Conflict of interest statement
JF is an employee of Affibody AB and CMC director for the ABY-025 program. Affibody AB holds the intellectual property rights and trademarks for Affibody molecules. The remaining authors have no competing interests to declare.
Figures





Similar articles
-
Diagnostic HER2-binding radiopharmaceutical, [68Ga]Ga-ABY-025, for routine clinical use in breast cancer patients.Am J Nucl Med Mol Imaging. 2019 Feb 15;9(1):12-23. eCollection 2019. Am J Nucl Med Mol Imaging. 2019. PMID: 30911434 Free PMC article.
-
A HER2-binding Affibody molecule labelled with 68Ga for PET imaging: direct in vivo comparison with the 111In-labelled analogue.Eur J Nucl Med Mol Imaging. 2010 Jul;37(7):1356-67. doi: 10.1007/s00259-009-1367-7. Epub 2010 Feb 4. Eur J Nucl Med Mol Imaging. 2010. PMID: 20130858
-
Human Epidermal Growth Factor Receptor 2-Targeting [68Ga]Ga-ABY-025 PET/CT Predicts Early Metabolic Response in Metastatic Breast Cancer.J Nucl Med. 2023 Sep;64(9):1364-1370. doi: 10.2967/jnumed.122.265364. Epub 2023 Jul 13. J Nucl Med. 2023. PMID: 37442602 Free PMC article. Clinical Trial.
-
Measuring HER2-Receptor Expression In Metastatic Breast Cancer Using [68Ga]ABY-025 Affibody PET/CT.Theranostics. 2016 Jan 1;6(2):262-71. doi: 10.7150/thno.13502. eCollection 2016. Theranostics. 2016. PMID: 26877784 Free PMC article.
-
Human Epidermal Growth Factor Receptor 2 (HER2) PET Imaging of HER2-Low Breast Cancer with [68Ga]Ga-ABY-025: Results from a Pilot Study.J Nucl Med. 2024 May 1;65(5):700-707. doi: 10.2967/jnumed.123.266847. J Nucl Med. 2024. PMID: 38548353
References
-
- Frane N, Bitterman A (2023) Radiation Safety and Protection. StatPearls. Treasure Island (FL) ineligible companies. Disclosure: Adam Bitterman declares no relevant financial relationships with ineligible companies.: StatPearls Publishing. Copyright © 2023, StatPearls Publishing LLC.; 2023. - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
