Expert Consensus on SABA Use for Asthma Clinical Decision-Making: A Delphi Approach
- PMID: 37991672
- PMCID: PMC10716188
- DOI: 10.1007/s11882-023-01111-z
Expert Consensus on SABA Use for Asthma Clinical Decision-Making: A Delphi Approach
Abstract
Purpose of review: A modified Delphi process was undertaken to provide a US expert-led consensus to guide clinical action on short-acting beta2-agonist (SABA) use. This comprised an online survey (Phase 1), forum discussion and statement development (Phase 2), and statement adjudication (Phase 3).
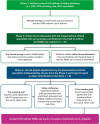
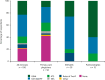
Recent findings: In Phase 1 (n = 100 clinicians), 12% routinely provided patients with ≥4 SABA prescriptions/year, 73% solicited SABA use frequency at every patient visit, and 21% did not consult asthma guidelines/expert reports. Phase 3 experts (n = 8) reached consensus (median Likert score, interquartile range) that use of ≥3 SABA canisters/year is associated with increased risk of exacerbation and asthma-related death (5, 4.75-5); SABA use history should be solicited at every patient visit (5, 4.75-5); usage patterns over time, not absolute thresholds, should guide response to SABA overuse (5, 4.5-5). Future asthma guidelines should include clear recommendations regarding SABA usage, using expert-led thresholds for action.
Keywords: Asthma; Asthma guidelines; Delphi consensus; Exacerbations; Reliever inhaler; SABA overuse.
© 2023. The Author(s).
Conflict of interest statement
N. Lugogo received consulting fees from Amgen, AstraZeneca, Avillion, Genentech, GSK, Novartis, Regeneron, Sanofi, and Teva; honoraria for non-speakers bureau presentations from GSK and AstraZeneca; and travel support from Astra Zeneca; her institution received research support from Amgen, AstraZeneca, Avillion, Evidera, Gossamer Bio, Genentech, GSK, Regeneron, Sanofi, Novartis and Teva. She is an honorary faculty member of Observational and Pragmatic Research Institute but does not receive compensation for this role. M. O’Connor has received honoraria and/or consulting fees from AstraZeneca, BioCryst, CSL Behring, GSK, Pharming Technologies BV, Sanofi, and Teva Pharmaceuticals. M. George has received honoraria and/or consulting fees from AstraZeneca, Genentech, Regeneron, Sanofi, and Teva Pharmaceuticals. R. Merchant has received honoraria and/or consulting fees from AstraZeneca, Propeller Health, Teva Pharmaceuticals, and Sanofi. G. Bensch has received honoraria and/or consulting fees from Amgen, Aimimmune, AstraZeneca, Regeneron, Sanofi, and Teva Pharmaceuticals. J. Portnoy has received honoraria and/or consulting fees from BioCryst and Thermo Fisher. J. Oppenheimer has received honoraria and/or consulting fees from Amgen, Aquestive, AstraZeneca, Novartis, GSK, Sanofi, and Regeneron. M. Castro reports institutional grant funding from NIH, ALA, PCORI, AstraZeneca, GSK, Novartis, Pulmatrix, Sanofi-Aventis, and Shionogi. He receives consulting fees from Allakos, Arrowhead, Genentech, GSK, Merck, Novartis, Sanofi-Aventis, Teva and OM Pharma. He receives payment for speaker’s bureau activities for Amgen, AstraZeneca, Genentech, Regeneron, Sanofi-Aventis, and Teva. He receives royalties from Aer Therapeutics and Elsevier.
Figures

References
-
- Centers for Disease Control and Prevention. Most recent national asthma data. 2021. Accessed September 27, 2023. https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm.
-
- Azzi EA, Kritikos V, Peters MJ, Price DB, Srour P, Cvetkovski B, et al. Understanding reliever overuse in patients purchasing over-the-counter short-acting beta(2) agonists: an Australian community pharmacy-based survey. BMJ Open. 2019;9(8):e028995. doi: 10.1136/bmjopen-2019-028995. - DOI - PMC - PubMed
-
- Bloom CI, Cabrera C, Arnetorp S, Coulton K, Nan C, van der Valk RJP, et al. Asthma-related health outcomes associated with short-acting β(2)-agonist inhaler use: an observational UK study as part of the SABINA global program. Adv Ther. 2020;37(10):4190–208. doi: 10.1007/s12325-020-01444-5. - DOI - PubMed
-
- •• Lugogo N, Gilbert I, Tkacz J, Gandhi H, Goshi N, Lanz MJ. Real-world patterns and implications of short-acting β(2)-agonist use in patients with asthma in the United States. Ann Allergy Asthma Immunol. 2021; 126(6):681-9.e1. Analysis of administrative claims data from 135,540 US asthma patients found that exacerbation risk increased with increasing SABA fills. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

