A Patient Charter for Chronic Urticaria
- PMID: 37991694
- PMCID: PMC10796664
- DOI: 10.1007/s12325-023-02724-6
A Patient Charter for Chronic Urticaria
Abstract
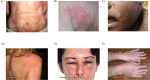
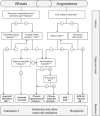
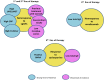
Chronic urticaria (CU) is the recurring development of wheals (aka "hives" or "welts"), angioedema, or both for more than 6 weeks. Wheals and angioedema occur with no definite triggers in chronic spontaneous urticaria, and in response to known and definite physical triggers in chronic inducible urticaria. Approximately 1.4% of individuals globally will have CU during their lifetime. The itching and physical discomfort associated with CU have a profound impact on daily activities, sexual function, work or school performance, and sleep, causing significant impairment in a patient's physical and mental quality of life. CU also places a financial burden on patients and healthcare systems. Patients should feel empowered to self-advocate to receive the best care. The voice of the patient in navigating the journey of CU diagnosis and management may improve patient-provider communication, thereby improving diagnosis and outcomes. A collaboration of patients, providers, advocacy organizations, and pharmaceutical representatives have created a patient charter to define the realistic and achievable principles of care that patients with CU should expect to receive. Principle (1): I deserve an accurate and timely diagnosis of my CU; Principle (2): I deserve access to specialty care for my CU; Principle (3): I deserve access to innovative treatments that reduce the burden of CU on my daily life; Principle (4): I deserve to be free of unnecessary treatment-related side-effects during the management of my CU; and Principle (5): I expect a holistic treatment approach to address all the components of my life impacted by CU. The stated principles may serve as a guide for healthcare providers who care for patients with CU and translate into better patient-physician communication. In addition, we urge policymakers and authors of CU treatment guidelines to consider these principles in their decision-making to ensure the goals of the patient are achievable.
Keywords: Angioedema; Chronic urticaria; Health care; Hives; Patient advocacy; Wheals.
© 2023. The Author(s).
Conflict of interest statement
M. Maurer is or recently was a speaker and/or advisor for and/or has received research funding from Allakos, Alvotech, Amgen, Aquestive, Aralez, AstraZeneca, Bayer, Celldex, Celltrion, Evommune, GSK, Ipsen, Kyowa Kirin, Leo Pharma, Lilly, Menarini, Mitsubishi Tanabe Pharma, Moxie, Noucor, Novartis, Orion Biotechnology, Resoncance Medicine, Sanofi/Regeneron, Septerna, Trial Form Support International AB, Third HarmonicBio, ValenzaBio, Yuhan Corporation, Zurabio. M. Albuquerque has nothing to disclose. J-N. Boursiquot has nothing to disclose. E. Dery has nothing to disclose. A. Giménez-Arnau is or recently was a speaker and/or advisor for and/or has received research funding from Almirall, Amgen, AstraZeneca, Avene, Celldex, Escient Pharmaceutials, Genentech, GSK, Instituto Carlos III- FEDER, Leo Pharma, Menarini, Mitsubishi Tanabe Pharma, Novartis, Sanofi–Regeneron, Servier, Thermo Fisher Scientific, Uriach Pharma / Neucor. K. Godse has nothing to disclose. G. Guitiérrez has nothing to disclose. A. Kanani has nothing to disclose. G Lacuesta has received industry sponsored funding from Novartis, Astra Zeneca, Takeda; received speakers fees/advisory board fees from Novartis, Astra Zeneca, GSK, Sanofi, Abbvie, CSL Behring, Takeda. J. McCarthy is an employee of Novartis Pharmaceutical Corporation, East Hanover, NJ, USA. S. Nigen has nothing to disclose. T. Winders has nothing to disclose.
Figures



References
-
- Sánchez-Borges M, Ansotegui IJ, Baiardini I, Bernstein J, Canonica GW, Ebisawa M, et al. The challenges of chronic urticaria part 1: epidemiology, immunopathogenesis, comorbidities, quality of life, and management. World Allergy Organ J. 2021;14:100533. doi: 10.1016/j.waojou.2021.100533. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

