SUMOylation-Driven mRNA Circularization Enhances Translation and Promotes Lymphatic Metastasis of Bladder Cancer
- PMID: 37991737
- PMCID: PMC10831341
- DOI: 10.1158/0008-5472.CAN-23-2278
SUMOylation-Driven mRNA Circularization Enhances Translation and Promotes Lymphatic Metastasis of Bladder Cancer
Abstract
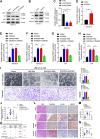
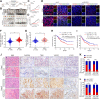
Aberrant gene expression is a prominent feature of metastatic cancer. Translational initiation is a vital step in fine-tuning gene expression. Thus, exploring translation initiation regulators may identify therapeutic targets for preventing and treating metastasis. Herein, we identified that DHCR24 was overexpressed in lymph node (LN) metastatic bladder cancer and correlated with poor prognosis of patients. DHCR24 promoted lymphangiogenesis and LN metastasis of bladder cancer in vitro and in vivo. Mechanistically, DHCR24 mediated and recognized the SUMO2 modification at lysine 108 of hnRNPA2B1 to foster TBK1 mRNA circularization and eIF4F initiation complex assembly by enhancing hnRNPA2B1-eIF4G1 interaction. Moreover, DHCR24 directly anchored to TBK1 mRNA 3'-untranslated region to increase its stability, thus forming a feed forward loop to elevate TBK1 expression. TBK1 activated PI3K/Akt signaling to promote VEGFC secretion, resulting in lymphangiogenesis and LN metastasis. DHCR24 silencing significantly impeded bladder cancer lymphangiogenesis and lymphatic metastasis in a patient-derived xenograft model. Collectively, these findings elucidate DHCR24-mediated translation machinery that promotes lymphatic metastasis of bladder cancer and supports the potential application of DHCR24-targeted therapy for LN-metastatic bladder cancer.
Significance: DHCR24 is a SUMOylation regulator that controls translation initiation complex assembly and orchestrates TBK1 mRNA circularization to activate Akt/VEGFC signaling, which stimulates lymphangiogenesis and promotes lymph node metastasis in bladder cancer.
©2023 The Authors; Published by the American Association for Cancer Research.
Figures





References
-
- Hautmann RE, de Petriconi RC, Pfeiffer C, Volkmer BG. Radical cystectomy for urothelial carcinoma of the bladder without neoadjuvant or adjuvant therapy: long-term results in 1,100 patients. Eur Urol 2012;61:1039–47. - PubMed
-
- Zahoor H, Mir MC, Barata PC, Stephenson AJ, Campbell SC, Fergany A, et al. Phase II trial of continuous treatment with sunitinib in patients with high-risk (BCG-refractory) non-muscle invasive bladder cancer. Invest New Drugs 2019;37:1231–8. - PubMed
-
- Sun L, Wang W, Han C, Huang W, Sun Y, Fang K, et al. The oncomicropeptide APPLE promotes hematopoietic malignancy by enhancing translation initiation. Mol Cell 2021;81:4493–508e9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 2022YFA1305500/National Key Research and Development Program of China (NKPs)
- 2018YFA0902803/National Key Research and Development Program of China (NKPs)
- 32322023/National Natural Science Foundation of China (NSFC)
- 82202276/National Natural Science Foundation of China (NSFC)
- 82072639/National Natural Science Foundation of China (NSFC)
- 81802530/National Natural Science Foundation of China (NSFC)
- 81871945/National Natural Science Foundation of China (NSFC)
- 81702951/National Natural Science Foundation of China (NSFC)
- 81672395/National Natural Science Foundation of China (NSFC)
- 81672807/National Natural Science Foundation of China (NSFC)
- 81702417/National Natural Science Foundation of China (NSFC)
- 81701715/National Natural Science Foundation of China (NSFC)
- 2022A1515012288/Natural Science Foundation of Guangdong Province ()
- 2021A1515010355/Natural Science Foundation of Guangdong Province ()
- 2022B1515120086/Natural Science Foundation of Guangdong Province ()
- 2020A1515010815/Natural Science Foundation of Guangdong Province ()
- 2018A030313564/Natural Science Foundation of Guangdong Province ()
- 2017A020215072/Natural Science Foundation of Guangdong Province ()
- 2016A030313340/Natural Science Foundation of Guangdong Province ()
- 2016A030313296/Natural Science Foundation of Guangdong Province ()
- 2017A030313880/Natural Science Foundation of Guangdong Province ()
- 2017A030310200/Natural Science Foundation of Guangdong Province ()
- 2023A04J2206/Scientific and Technological Planning Project of Guangzhou City ()
- 202002030388/Scientific and Technological Planning Project of Guangzhou City ()
- 201803010049/Scientific and Technological Planning Project of Guangzhou City ()
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

