Abbreviated or Standard Dual Antiplatelet Therapy by Sex in Patients at High Bleeding Risk: A Prespecified Secondary Analysis of a Randomized Clinical Trial
- PMID: 37991745
- PMCID: PMC10666042
- DOI: 10.1001/jamacardio.2023.4316
Abbreviated or Standard Dual Antiplatelet Therapy by Sex in Patients at High Bleeding Risk: A Prespecified Secondary Analysis of a Randomized Clinical Trial
Abstract
Importance: Abbreviated dual antiplatelet therapy (DAPT) reduces bleeding with no increase in ischemic events in patients at high bleeding risk (HBR) undergoing percutaneous coronary intervention (PCI).
Objectives: To evaluate the association of sex with the comparative effectiveness of abbreviated vs standard DAPT in patients with HBR.
Design, setting, and patients: This prespecified subgroup comparative effectiveness analysis followed the Management of High Bleeding Risk Patients Post Bioresorbable Polymer Coated Stent Implantation With an Abbreviated vs Standard DAPT Regimen (MASTER DAPT) trial, a multicenter, randomized, open-label clinical trial conducted at 140 sites in 30 countries and performed from February 28, 2017, to December 5, 2019. A total of 4579 patients with HBR were randomized at 1 month after PCI to abbreviated or standard DAPT. Data were analyzed from July 1 to October 31, 2022.
Interventions: Abbreviated (immediate DAPT discontinuation, followed by single APT for ≥6 months) or standard (DAPT for ≥2 additional months, followed by single APT for 11 months) treatment groups.
Main outcomes and measures: One-year net adverse clinical events (NACEs) (a composite of death due to any cause, myocardial infarction, stroke, or major bleeding), major adverse cardiac or cerebral events (MACCEs) (a composite of death due to any cause, myocardial infarction, or stroke), and major or clinically relevant nonmajor bleeding (MCB).
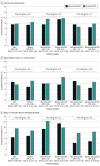
Results: Of the 4579 patients included in the analysis, 1408 (30.7%) were women and 3171 (69.3%) were men (mean [SD] age, 76.0 [8.7] years). Ischemic and bleeding events were similar between sexes. Abbreviated DAPT was associated with comparable NACE rates in men (hazard ratio [HR], 0.97 [95% CI, 0.75-1.24]) and women (HR, 0.87 [95% CI, 0.60-1.26]; P = .65 for interaction). There was evidence of heterogeneity of treatment effect by sex for MACCEs, with a trend toward benefit in women (HR, 0.68 [95% CI, 0.44-1.05]) but not in men (HR, 1.17 [95% CI, 0.88-1.55]; P = .04 for interaction). There was no significant interaction for MCB across sex, although the benefit with abbreviated DAPT was relatively greater in men (HR, 0.65 [95% CI, 0.50-0.84]) than in women (HR, 0.77 [95% CI, 0.53-1.12]; P = .46 for interaction). Results remained consistent in patients with acute coronary syndrome and/or complex PCI.
Conclusions and relevance: These findings suggest that women with HBR did not experience higher rates of ischemic or bleeding events compared with men and may derive particular benefit from abbreviated compared with standard DAPT owing to these numerically lower rates of events.
Trial registration: ClinicalTrials.gov Identifier: NCT03023020.
Conflict of interest statement
Figures


References
-
- Valgimigli M, Costa F, Lokhnygina Y, et al. Trade-off of myocardial infarction vs. bleeding types on mortality after acute coronary syndrome: lessons from the Thrombin Receptor Antagonist for Clinical Event Reduction in Acute Coronary Syndrome (TRACER) randomized trial. Eur Heart J. 2017;38(11):804-810. doi: 10.1093/eurheartj/ehw525 - DOI - PMC - PubMed
-
- Ueki Y, Bär S, Losdat S, et al. Validation of the Academic Research Consortium for High Bleeding Risk (ARC-HBR) criteria in patients undergoing percutaneous coronary intervention and comparison with contemporary bleeding risk scores. EuroIntervention. 2020;16(5):371-379. doi: 10.4244/EIJ-D-20-00052 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

