BMP9 is a potent inducer of chondrogenesis, volumetric expansion and collagen type II accumulation in bovine auricular cartilage chondroprogenitors
- PMID: 37992123
- PMCID: PMC10664884
- DOI: 10.1371/journal.pone.0294761
BMP9 is a potent inducer of chondrogenesis, volumetric expansion and collagen type II accumulation in bovine auricular cartilage chondroprogenitors
Abstract
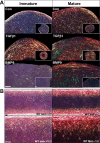
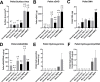
Reconstruction of the outer ear currently requires harvesting of cartilage from the posterior of the auricle or ribs leading to pain and donor site morbidity. An alternative source for auricular reconstruction is in vitro tissue engineered cartilage using stem/progenitor cells. Several candidate cell-types have been studied with tissue-specific auricular cartilage progenitor cells (AuCPC) of particular interest. Whilst chondrogenic differentiation of competent stem cells using growth factor TGFβ1 produces cartilage this tissue is frequently fibrocartilaginous and lacks the morphological features of hyaline cartilage. Recent work has shown that growth factor BMP9 is a potent chondrogenic and morphogenetic factor for articular cartilage progenitor cells, and we hypothesised that this property extends to cartilage-derived progenitors from other tissues. In this study we show monoclonal populations of AuCPCs from immature and mature bovine cartilage cultured with BMP9 produced cartilage pellets have 3-5-fold greater surface area in sections than those grown with TGFβ1. Increased volumetric growth using BMP9 was due to greater sGAG deposition in immature pellets and significantly greater collagen accumulation in both immature and mature progenitor pellets. Polarised light microscopy and immunohistochemical analyses revealed that the organisation of collagen fibrils within pellets is an important factor in the growth of pellets. Additionally, chondrocytes in BMP9 stimulated cell pellets had larger lacunae and were more evenly dispersed throughout the extracellular matrix. Interestingly, BMP9 tended to normalise the response of immature AuCPC monoclonal cell lines to differentiation cues whereas cells exhibited more variation under TGFβ1. In conclusion, BMP9 appears to be a potent inducer of chondrogenesis and volumetric growth for AuCPCs a property that can be exploited for tissue engineering strategies for reconstructive surgery though with the caveat of negligible elastin production following 21-day treatment with either growth factor.
Copyright: © 2023 Gardner et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Bone Morphogenetic Protein-9 Is a Potent Chondrogenic and Morphogenic Factor for Articular Cartilage Chondroprogenitors.Stem Cells Dev. 2020 Jul;29(14):882-894. doi: 10.1089/scd.2019.0209. Epub 2020 May 28. Stem Cells Dev. 2020. PMID: 32364057 Free PMC article.
-
Progenitor cells in auricular cartilage demonstrate cartilage-forming capacity in 3D hydrogel culture.Eur Cell Mater. 2018 Feb 27;35:132-150. doi: 10.22203/eCM.v035a10. Eur Cell Mater. 2018. PMID: 29485180
-
Facilitating In Vivo Articular Cartilage Repair by Tissue-Engineered Cartilage Grafts Produced From Auricular Chondrocytes.Am J Sports Med. 2018 Mar;46(3):713-727. doi: 10.1177/0363546517741306. Epub 2017 Dec 6. Am J Sports Med. 2018. PMID: 29211970
-
Reserve or Resident Progenitors in Cartilage? Comparative Analysis of Chondrocytes versus Chondroprogenitors and Their Role in Cartilage Repair.Cartilage. 2018 Apr;9(2):171-182. doi: 10.1177/1947603517736108. Epub 2017 Oct 19. Cartilage. 2018. PMID: 29047310 Free PMC article.
-
The use of mesenchymal stem cells for chondrogenesis.Injury. 2008 Apr;39 Suppl 1:S58-65. doi: 10.1016/j.injury.2008.01.038. Injury. 2008. PMID: 18313473 Review.
Cited by
-
BMP9 induces postnatal zonal stratification of immature articular cartilage through reconfiguration of the existing collagen framework.Front Cell Dev Biol. 2025 Jan 28;12:1511908. doi: 10.3389/fcell.2024.1511908. eCollection 2024. Front Cell Dev Biol. 2025. PMID: 39935787 Free PMC article.
-
Cartilage Organoids from Articular Chondroprogenitor Cells and Their Potential to Produce Neo-Hyaline Cartilage.Cartilage. 2025 Feb 10:19476035241313179. doi: 10.1177/19476035241313179. Online ahead of print. Cartilage. 2025. PMID: 39925233 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

