What makes a winner? Symbiont and host dynamics determine Caribbean octocoral resilience to bleaching
- PMID: 37992160
- PMCID: PMC10664981
- DOI: 10.1126/sciadv.adj6788
What makes a winner? Symbiont and host dynamics determine Caribbean octocoral resilience to bleaching
Abstract
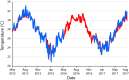
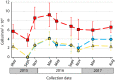
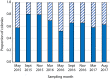
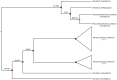
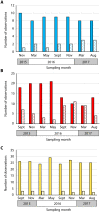
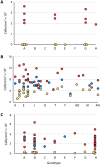
Unlike reef-building, scleractinian corals, Caribbean soft corals (octocorals) have not suffered marked declines in abundance associated with anthropogenic ocean warming. Both octocorals and reef-building scleractinians depend on a nutritional symbiosis with single-celled algae living within their tissues. In both groups, increased ocean temperatures can induce symbiont loss (bleaching) and coral death. Multiple heat waves from 2014 to 2016 resulted in widespread damage to reef ecosystems and provided an opportunity to examine the bleaching response of three Caribbean octocoral species. Symbiont densities declined during the heat waves but recovered quickly, and colony mortality was low. The dominant symbiont genotypes within a host generally did not change, and all colonies hosted symbiont species in the genus Breviolum. Their association with thermally tolerant symbionts likely contributes to the octocoral holobiont's resistance to mortality and the resilience of their symbiont populations. The resistance and resilience of Caribbean octocorals offer clues for the future of coral reefs.
Figures

References
-
- M. L. Reaka-Kudla, Known and unknown biodiversity, risk of extinction and conservation strategy in the sea, in Waters in Peril, L. Bendell-Young, P. Gallaugher, Eds. (Springer, 2001), pp. 19–33.
-
- R. Costanza, R. de Groot, P. Sutton, S. van der Ploeg, S. J. Anderson, I. Kubiszewski, S. Farber, R. K. Turner, Changes in the global value of ecosystem services. Glob. Environ. Change 26, 152–158 (2014).
-
- T. A. Gardner, I. M. Cote, J. A. Gill, A. Grant, A. R. Watkinson, Long-term region-wide declines in Caribbean corals. Science 301, 958–960 (2003). - PubMed
-
- O. Hoegh-Guldberg, Climate change, coral bleaching and the future of the world's coral reefs. Mar. Freshw. Res. 50, 839–866 (1999).
-
- P. G. Falkowski, Z. Dubinsky, L. Muscatine, J. W. Porter, Light and the bioenergetics of a symbiotic coral. Bioscience 34, 705–709 (1984).
MeSH terms
LinkOut - more resources
Full Text Sources

