Insulin-like growth factor binding protein-2 in at-risk adults and autopsy-confirmed Alzheimer brains
- PMID: 37992295
- PMCID: PMC11068109
- DOI: 10.1093/brain/awad398
Insulin-like growth factor binding protein-2 in at-risk adults and autopsy-confirmed Alzheimer brains
Abstract
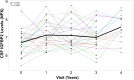
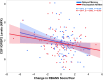
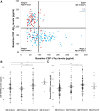
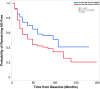
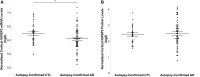
Insulin, insulin-like growth factors (IGF) and their receptors are highly expressed in the adult hippocampus. Thus, disturbances in the insulin-IGF signalling pathway may account for the selective vulnerability of the hippocampus to nascent Alzheimer's disease (AD) pathology. In the present study, we examined the predominant IGF-binding protein in the CSF, IGFBP2. CSF was collected from 109 asymptomatic members of the parental history-positive PREVENT-AD cohort. CSF levels of IGFBP2, core AD and synaptic biomarkers were measured using proximity extension assay, ELISA and mass spectrometry. Cortical amyloid-beta (Aβ) and tau deposition were examined using 18F-NAV4694 and flortaucipir. Cognitive assessments were performed during up to 8 years of follow-up, using the Repeatable Battery for the Assessment of Neuropsychological Status. T1-weighted structural MRI scans were acquired, and neuroimaging analyses were performed on pre-specified temporal and parietal brain regions. Next, in an independent cohort, we allocated 241 dementia-free ADNI-1 participants into four stages of AD progression based on the biomarkers CSF Aβ42 and total-tau (t-tau). In this analysis, differences in CSF and plasma IGFBP2 levels were examined across the pathological stages. Finally, IGFBP2 mRNA and protein levels were examined in the frontal cortex of 55 autopsy-confirmed AD and 31 control brains from the Quebec Founder Population (QFP) cohort, a unique population isolated from Eastern Canada. CSF IGFBP2 progressively increased over 5 years in asymptomatic PREVENT-AD participants. Baseline CSF IGFBP2 was positively correlated with CSF AD biomarkers and synaptic biomarkers, and negatively correlated with longitudinal changes in delayed memory (P = 0.024) and visuospatial abilities (P = 0.019). CSF IGFBP2 was negatively correlated at a trend-level with entorhinal cortex volume (P = 0.082) and cortical thickness in the piriform (P = 0.039), inferior temporal (P = 0.008), middle temporal (P = 0.014) and precuneus (P = 0.033) regions. In ADNI-1, CSF (P = 0.009) and plasma (P = 0.001) IGFBP2 were significantly elevated in Stage 2 [CSF Aβ(+)/t-tau(+)]. In survival analyses in ADNI-1, elevated plasma IGFBP2 was associated with a greater rate of AD conversion (hazard ratio = 1.62, P = 0.021). In the QFP cohort, IGFBP2 mRNA was reduced (P = 0.049); however, IGFBP2 protein levels did not differ in the frontal cortex of autopsy-confirmed AD brains (P = 0.462). Nascent AD pathology may induce an upregulation in IGFBP2 in asymptomatic individuals. CSF and plasma IGFBP2 may be valuable markers for identifying CSF Aβ(+)/t-tau(+) individuals and those with a greater risk of AD conversion.
Keywords: Alzheimer's disease; RBANS; cerebrospinal fluid; insulin-like growth factor; insulin-like growth factor-binding protein-2; post-mortem brain tissue.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
Conflict of interest statement
J.P. serves as a scientific advisor to the Alzheimer Society of France. H.Z. has served at scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZPath, Annexon, Apellis, Artery Therapeutics, AZTherapies, CogRx, Denali, Eisai, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave, has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure, Biogen and Roche, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is part of the GU Ventures Incubator Program (outside submitted work). K.B. has served as a consultant and at advisory boards for Acumen, ALZPath, BioArctic, Biogen, Eisai, Lilly, Moleac Pte. Ltd, Novartis, Ono Pharma, Prothena, Roche Diagnostics and Siemens Healthineers; has served at data monitoring committees for Julius Clinical and Novartis; has given lectures, produced educational materials and participated in educational programs for AC Immune, Biogen, Celdara Medical, Eisai and Roche Diagnostics; and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program, outside the work presented in this paper.
Figures


Comment in
-
IGFBP2 levels associated with Alzheimer disease.Nat Rev Neurol. 2024 Jan;20(1):1. doi: 10.1038/s41582-023-00911-1. Nat Rev Neurol. 2024. PMID: 38049639 No abstract available.
References
-
- Alzheimer’s Association . 2022 Alzheimer’s disease facts and figures. Alzheimers Dement. 2022;18:700–789. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

