Circulating tumor DNA and radiological tumor volume identify patients at risk for relapse with resected, early-stage non-small-cell lung cancer
- PMID: 37992871
- PMCID: PMC11233158
- DOI: 10.1016/j.annonc.2023.11.008
Circulating tumor DNA and radiological tumor volume identify patients at risk for relapse with resected, early-stage non-small-cell lung cancer
Abstract
Background: Predicting relapse and overall survival (OS) in early-stage non-small-cell lung cancer (NSCLC) patients remains challenging. Therefore, we hypothesized that detection of circulating tumor DNA (ctDNA) can identify patients with increased risk of relapse and that integrating radiological tumor volume measurement along with ctDNA detectability improves prediction of outcome.
Patients and methods: We analyzed 366 serial plasma samples from 85 patients who underwent surgical resections and assessed ctDNA using a next-generation sequencing liquid biopsy assay, and measured tumor volume using a computed tomography-based three-dimensional annotation.
Results: Our results showed that patients with detectable ctDNA at baseline or after treatment and patients who did not clear ctDNA after treatment had a significantly worse clinical outcome. Integrating radiological analysis allowed the stratification in risk groups prognostic of clinical outcome as confirmed in an independent cohort of 32 patients.
Conclusions: Our findings suggest ctDNA and radiological monitoring could be valuable tools for guiding follow-up care and treatment decisions for early-stage NSCLC patients.
Keywords: MRD; ctDNA; early-stage NSCLC; liquid biopsy; tumor volume.
Copyright © 2023 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures
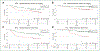


References
-
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. - PubMed
-
- Pantel K, Alix-Panabières C. Liquid biopsy and minimal residual disease — latest advances and implications for cure. Nat Rev Clin Oncol. 2019;16(7):409–424. - PubMed
-
- Tarazona N, Gimeno-Valiente F, Gambardella V, et al. Targeted next-generation sequencing of circulating-tumor DNA for tracking minimal residual disease in localized colon cancer. Ann Oncol. 2019;30(11): 1804–1812. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

