Megakaryocytes possess a STING pathway that is transferred to platelets to potentiate activation
- PMID: 37993259
- PMCID: PMC10665521
- DOI: 10.26508/lsa.202302211
Megakaryocytes possess a STING pathway that is transferred to platelets to potentiate activation
Abstract
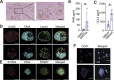
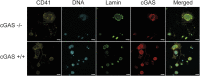
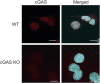
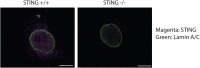
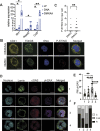
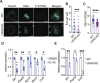
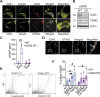
Platelets display unexpected roles in immune and coagulation responses. Emerging evidence suggests that STING is implicated in hypercoagulation. STING is an adaptor protein downstream of the DNA sensor cyclic GMP-AMP synthase (cGAS) that is activated by cytosolic microbial and self-DNA during infections, and in the context of loss of cellular integrity, to instigate the production of type-I IFN and pro-inflammatory cytokines. To date, whether the cGAS-STING pathway is present in platelets and contributes to platelet functions is not defined. Using a combination of pharmacological and genetic approaches, we demonstrate here that megakaryocytes and platelets possess a functional cGAS-STING pathway. Our results suggest that in megakaryocytes, STING stimulation activates a type-I IFN response, and during thrombopoiesis, cGAS and STING are transferred to proplatelets. Finally, we show that both murine and human platelets contain cGAS and STING proteins, and the cGAS-STING pathway contributes to potentiation of platelet activation and aggregation. Taken together, these observations establish for the first time a novel role of the cGAS-STING DNA sensing axis in the megakaryocyte and platelet lineage.
© 2023 El-Mortada et al.
Conflict of interest statement
M Lordkipanidzé has received speaker honoraria from Bayer and JAMP Pharma; has received research grants to the institution from Idorsia; has served on a national advisory board for Servier and JAMP Pharma; and has received in-kind support for investigator-initiated grants from Fujimori Kogyo. There are no other conflicts of interest to disclose.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
