Neuroblastoma: an ongoing cold front for cancer immunotherapy
- PMID: 37993280
- PMCID: PMC10668262
- DOI: 10.1136/jitc-2023-007798
Neuroblastoma: an ongoing cold front for cancer immunotherapy
Abstract
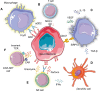
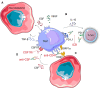
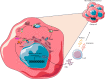
Neuroblastoma is the most frequent extracranial childhood tumour but effective treatment with current immunotherapies is challenging due to its immunosuppressive microenvironment. Efforts to date have focused on using immunotherapy to increase tumour immunogenicity and enhance anticancer immune responses, including anti-GD2 antibodies; immune checkpoint inhibitors; drugs which enhance macrophage and natural killer T (NKT) cell function; modulation of the cyclic GMP-AMP synthase-stimulator of interferon genes pathway; and engineering neuroblastoma-targeting chimeric-antigen receptor-T cells. Some of these strategies have strong preclinical foundation and are being tested clinically, although none have demonstrated notable success in treating paediatric neuroblastoma to date. Recently, approaches to overcome heterogeneity of neuroblastoma tumours and treatment resistance are being explored. These include rational combination strategies with the aim of achieving synergy, such as dual targeting of GD2 and tumour-associated macrophages or natural killer cells; GD2 and the B7-H3 immune checkpoint; GD2 and enhancer of zeste-2 methyltransferase inhibitors. Such combination strategies provide opportunities to overcome primary resistance to and maximize the benefits of immunotherapy in neuroblastoma.
Keywords: Immunotherapy; Neuroblastoma; Tumor Microenvironment.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
