A distinct topology of BTN3A IgV and B30.2 domains controlled by juxtamembrane regions favors optimal human γδ T cell phosphoantigen sensing
- PMID: 37993425
- PMCID: PMC10665462
- DOI: 10.1038/s41467-023-41938-8
A distinct topology of BTN3A IgV and B30.2 domains controlled by juxtamembrane regions favors optimal human γδ T cell phosphoantigen sensing
Abstract
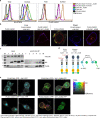
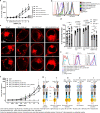
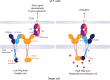
Butyrophilin (BTN)-3A and BTN2A1 molecules control the activation of human Vγ9Vδ2 T cells during T cell receptor (TCR)-mediated sensing of phosphoantigens (PAg) derived from microbes and tumors. However, the molecular rules governing PAg sensing remain largely unknown. Here, we establish three mechanistic principles of PAg-mediated γδ T cell activation. First, in humans, following PAg binding to the intracellular BTN3A1-B30.2 domain, Vγ9Vδ2 TCR triggering involves the extracellular V-domain of BTN3A2/BTN3A3. Moreover, the localization of both protein domains on different chains of the BTN3A homo-or heteromers is essential for efficient PAg-mediated activation. Second, the formation of BTN3A homo-or heteromers, which differ in intracellular trafficking and conformation, is controlled by molecular interactions between the juxtamembrane regions of the BTN3A chains. Finally, the ability of PAg not simply to bind BTN3A-B30.2, but to promote its subsequent interaction with the BTN2A1-B30.2 domain, is essential for T-cell activation. Defining these determinants of cooperation and the division of labor in BTN proteins improves our understanding of PAg sensing and elucidates a mode of action that may apply to other BTN family members.
© 2023. The Author(s).
Conflict of interest statement
B.E.W. provides consultancy regarding the development of γδ T cell immunotherapy approaches for Ferring Ventures SA, linked to Ferring Pharmaceuticals. T.H. is supported by Byondis B.V. for work not related to this study.
Figures



Update of
-
Division of labor and cooperation between different butyrophilin proteins controls phosphoantigen-mediated activation of human γδ T cells.Res Sq [Preprint]. 2023 Feb 15:rs.3.rs-2583246. doi: 10.21203/rs.3.rs-2583246/v1. Res Sq. 2023. Update in: Nat Commun. 2023 Nov 22;14(1):7617. doi: 10.1038/s41467-023-41938-8. PMID: 36824912 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

