Mirabegron displays anticancer effects by globally browning adipose tissues
- PMID: 37993438
- PMCID: PMC10665320
- DOI: 10.1038/s41467-023-43350-8
Mirabegron displays anticancer effects by globally browning adipose tissues
Abstract
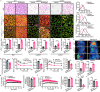
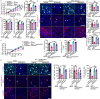
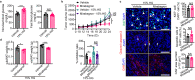
Metabolic reprogramming in malignant cells is a hallmark of cancer that relies on augmented glycolytic metabolism to support their growth, invasion, and metastasis. However, the impact of global adipose metabolism on tumor growth and the drug development by targeting adipose metabolism remain largely unexplored. Here we show that a therapeutic paradigm of drugs is effective for treating various cancer types by browning adipose tissues. Mirabegron, a clinically available drug for overactive bladders, displays potent anticancer effects in various animal cancer models, including untreatable cancers such as pancreatic ductal adenocarcinoma and hepatocellular carcinoma, via the browning of adipose tissues. Genetic deletion of the uncoupling protein 1, a key thermogenic protein in adipose tissues, ablates the anticancer effect. Similarly, the removal of brown adipose tissue, which is responsible for non-shivering thermogenesis, attenuates the anticancer activity of mirabegron. These findings demonstrate that mirabegron represents a paradigm of anticancer drugs with a distinct mechanism for the effective treatment of multiple cancers.
© 2023. The Author(s).
Conflict of interest statement
All authors claim no conflict of interest related to this work.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

