Clinical, genetic and structural delineation of RPL13-related spondyloepimetaphyseal dysplasia suggest extra-ribosomal functions of eL13
- PMID: 37993442
- PMCID: PMC10665555
- DOI: 10.1038/s41525-023-00380-x
Clinical, genetic and structural delineation of RPL13-related spondyloepimetaphyseal dysplasia suggest extra-ribosomal functions of eL13
Abstract
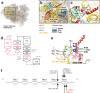
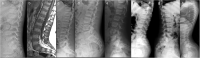
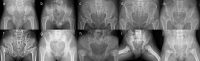
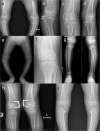
Spondyloepimetaphyseal dysplasia with severe short stature, RPL13-related (SEMD-RPL13), MIM#618728), is a rare autosomal dominant disorder characterized by short stature and skeletal changes such as mild spondylar and epimetaphyseal dysplasia affecting primarily the lower limbs. The genetic cause was first reported in 2019 by Le Caignec et al., and six disease-causing variants in the gene coding for a ribosomal protein, RPL13 (NM_000977.3) have been identified to date. This study presents clinical and radiographic data from 12 affected individuals aged 2-64 years from seven unrelated families, showing highly variable manifestations. The affected individuals showed a range from mild to severe short stature, retaining the same radiographic pattern of spondylar- and epi-metaphyseal dysplasia, but with varying severity of the hip and knee deformities. Two new missense variants, c.548 G>A, p.(Arg183His) and c.569 G>T, p.(Arg190Leu), and a previously known splice variant c.477+1G>A were identified, confirming mutational clustering in a highly specific RNA binding motif. Structural analysis and interpretation of the variants' impact on the protein suggests that disruption of extra-ribosomal functions of the protein through binding of mRNA may play a role in the skeletal phenotype of SEMD-RPL13. In addition, we present gonadal and somatic mosaicism for the condition.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests. All the authors have read and approved the final manuscript.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources

