Phylogenetic comparative analysis of the cerebello-cerebral system in 34 species highlights primate-general expansion of cerebellar crura I-II
- PMID: 37993596
- PMCID: PMC10665558
- DOI: 10.1038/s42003-023-05553-z
Phylogenetic comparative analysis of the cerebello-cerebral system in 34 species highlights primate-general expansion of cerebellar crura I-II
Abstract
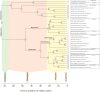
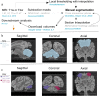
The reciprocal connections between the cerebellum and the cerebrum have been suggested to simultaneously play a role in brain size increase and to support a broad array of brain functions in primates. The cerebello-cerebral system has undergone marked functionally relevant reorganization. In particular, the lateral cerebellar lobules crura I-II (the ansiform) have been suggested to be expanded in hominoids. Here, we manually segmented 63 cerebella (34 primate species; 9 infraorders) and 30 ansiforms (13 species; 8 infraorders) to understand how their volumes have evolved over the primate lineage. Together, our analyses support proportional cerebellar-cerebral scaling, whereas ansiforms have expanded faster than the cerebellum and cerebrum. We did not find different scaling between strepsirrhines and haplorhines, nor between apes and non-apes. In sum, our study shows primate-general structural reorganization of the ansiform, relative to the cerebello-cerebral system, which is relevant for specialized brain functions in an evolutionary context.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests
Figures






References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

