Reduced LHFPL3-AS2 lncRNA expression is linked to altered epithelial polarity and proliferation, and to ileal ulceration in Crohn disease
- PMID: 37993670
- PMCID: PMC10665440
- DOI: 10.1038/s41598-023-47997-7
Reduced LHFPL3-AS2 lncRNA expression is linked to altered epithelial polarity and proliferation, and to ileal ulceration in Crohn disease
Abstract
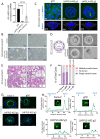
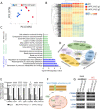
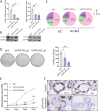
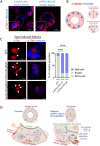
Disruption of intestinal epithelial functions is linked to Crohn disease (CD) pathogenesis. We identified a widespread reduction in the expression of long non-coding RNAs (lncRNAs) including LHFPL3-AS2 in the treatment-naïve CD ileum of the RISK pediatric cohort. We validated the reduction of LHFPL3-AS2 in adult CD and noted a further reduction in patients with more severe CD from the RISK cohort. LHFPL3-AS2 knockdown in Caco-2 cells robustly affected epithelial monolayer morphogenesis with markedly reduced confluency and spreading, showing atypical rounding, and clumping. mRNA-seq analysis of LHFPL3-AS2 knockdown cells highlighted the reduction of genes and pathways linked with apical polarity, actin bundles, morphogenesis, and the b-catenin-TCF4 complex. LHFPL3-AS2 knockdown significantly reduced the ability of cells to form an internal lumen within the 3-dimensional (3D) cyst model, with mislocalization of actin and adherent and tight junction proteins, affecting epithelial polarity. LHFPL3-AS2 knockdown also resulted in defective mitotic spindle formation and consequent reduction in epithelial proliferation. Altogether, we show that LHFPL3-AS2 reduction affects epithelial morphogenesis, polarity, mitotic spindle formation, and proliferation, which are key processes in maintaining epithelial homeostasis in CD. Reduced expression of LHFPL3-AS2 in CD patients and its further reduction with ileal ulceration outcome, emphasizes its significance in this context.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Long noncoding RNA LHFPL3-AS2 suppresses metastasis of non-small cell lung cancer by interacting with SFPQ to regulate TXNIP expression.Cancer Lett. 2022 Apr 10;531:1-13. doi: 10.1016/j.canlet.2022.01.031. Epub 2022 Jan 29. Cancer Lett. 2022. PMID: 35101541
-
Identification of LHFPL3-AS2 as a prognostic biomarker in lung adenocarcinoma.Indian J Pathol Microbiol. 2022 Apr-Jun;65(2):288-295. doi: 10.4103/IJPM.IJPM_1404_20. Indian J Pathol Microbiol. 2022. PMID: 35435361
-
Long ncRNA Landscape in the Ileum of Treatment-Naive Early-Onset Crohn Disease.Inflamm Bowel Dis. 2018 Jan 18;24(2):346-360. doi: 10.1093/ibd/izx013. Inflamm Bowel Dis. 2018. PMID: 29361088 Free PMC article.
-
ADAMTS9-AS2: A Functional Long Non-coding RNA in Tumorigenesis.Curr Pharm Des. 2021;27(23):2722-2727. doi: 10.2174/1381612827666210325105106. Curr Pharm Des. 2021. PMID: 33823762 Review.
-
Long Non-coding RNA DLGAP1-AS1 and DLGAP1-AS2: Two Novel Oncogenes in Multiple Cancers.Curr Med Chem. 2023;30(25):2822-2834. doi: 10.2174/0929867329666220919114919. Curr Med Chem. 2023. PMID: 36121088 Review.
Cited by
-
A Comprehensive Review on RNA Subcellular Localization Prediction.ArXiv [Preprint]. 2025 Apr 24:arXiv:2504.17162v1. ArXiv. 2025. PMID: 40313658 Free PMC article. Preprint.
-
The role of long non-coding RNA in Crohn's disease.Heliyon. 2024 Jun 6;10(11):e32606. doi: 10.1016/j.heliyon.2024.e32606. eCollection 2024 Jun 15. Heliyon. 2024. PMID: 38961978 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

