Sequential appetite suppression by oral and visceral feedback to the brainstem
- PMID: 37993711
- PMCID: PMC10700140
- DOI: 10.1038/s41586-023-06758-2
Sequential appetite suppression by oral and visceral feedback to the brainstem
Abstract
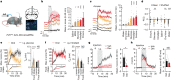
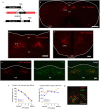
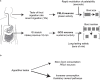
The termination of a meal is controlled by dedicated neural circuits in the caudal brainstem. A key challenge is to understand how these circuits transform the sensory signals generated during feeding into dynamic control of behaviour. The caudal nucleus of the solitary tract (cNTS) is the first site in the brain where many meal-related signals are sensed and integrated1-4, but how the cNTS processes ingestive feedback during behaviour is unknown. Here we describe how prolactin-releasing hormone (PRLH) and GCG neurons, two principal cNTS cell types that promote non-aversive satiety, are regulated during ingestion. PRLH neurons showed sustained activation by visceral feedback when nutrients were infused into the stomach, but these sustained responses were substantially reduced during oral consumption. Instead, PRLH neurons shifted to a phasic activity pattern that was time-locked to ingestion and linked to the taste of food. Optogenetic manipulations revealed that PRLH neurons control the duration of seconds-timescale feeding bursts, revealing a mechanism by which orosensory signals feed back to restrain the pace of ingestion. By contrast, GCG neurons were activated by mechanical feedback from the gut, tracked the amount of food consumed and promoted satiety that lasted for tens of minutes. These findings reveal that sequential negative feedback signals from the mouth and gut engage distinct circuits in the caudal brainstem, which in turn control elements of feeding behaviour operating on short and long timescales.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures














References
-
- Berthoud, H.-R. in Neurobiology of Food and Fluid Intake (eds Stricker, E. M. & Woods, S. C.) 195–240 (Springer, 2004).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

