Lactate receptor GPR81 drives breast cancer growth and invasiveness through regulation of ECM properties and Notch ligand DLL4
- PMID: 37993804
- PMCID: PMC10666402
- DOI: 10.1186/s12885-023-11631-6
Lactate receptor GPR81 drives breast cancer growth and invasiveness through regulation of ECM properties and Notch ligand DLL4
Abstract
Background: The lactate receptor GPR81 contributes to cancer development through unclear mechanisms. Here, we investigate the roles of GPR81 in three-dimensional (3D) and in vivo growth of breast cancer cells and study the molecular mechanisms involved.
Methods: GPR81 was stably knocked down (KD) in MCF-7 human breast cancer cells which were subjected to RNA-seq analysis, 3D growth, in situ- and immunofluorescence analyses, and cell viability- and motility assays, combined with KD of key GPR81-regulated genes. Key findings were additionally studied in other breast cancer cell lines and in mammary epithelial cells.
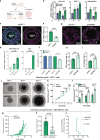
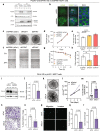
Results: GPR81 was upregulated in multiple human cancer types and further upregulated by extracellular lactate and 3D growth in breast cancer spheroids. GPR81 KD increased spheroid necrosis, reduced invasion and in vivo tumor growth, and altered expression of genes related to GO/KEGG terms extracellular matrix, cell adhesion, and Notch signaling. Single cell in situ analysis of MCF-7 cells revealed that several GPR81-regulated genes were upregulated in the same cell clusters. Notch signaling, particularly the Notch ligand Delta-like-4 (DLL4), was strikingly downregulated upon GPR81 KD, and DLL4 KD elicited spheroid necrosis and inhibited invasion in a manner similar to GPR81 KD.
Conclusions: GPR81 supports breast cancer aggressiveness, and in MCF-7 cells, this occurs at least in part via DLL4. Our findings reveal a new GPR81-driven mechanism in breast cancer and substantiate GPR81 as a promising treatment target.
Keywords: EPHA7; Extracellular matrix; HCAR1; Metabolite GPCR; Notch; PCDH7; Spheroid; Tumor microenvironment.
© 2023. The Author(s).
Conflict of interest statement
MT, TWS and SFP are co-founders of SOLID Therapeutics. All other authors declare no conflicts of interest.
Figures




Similar articles
-
The lactate receptor GPR81 promotes breast cancer growth via a paracrine mechanism involving antigen-presenting cells in the tumor microenvironment.Oncogene. 2020 Apr;39(16):3292-3304. doi: 10.1038/s41388-020-1216-5. Epub 2020 Feb 19. Oncogene. 2020. PMID: 32071396
-
Notch signaling mediated repressive effects of resveratrol in inducing caspasedependent apoptosis in MCF-7 breast cancer cells.Cell Mol Biol (Noisy-le-grand). 2024 Sep 8;70(8):96-103. doi: 10.14715/cmb/2024.70.8.12. Cell Mol Biol (Noisy-le-grand). 2024. PMID: 39262258
-
Anti-Cancer Effect of Melatonin via Downregulation of Delta-like Ligand 4 in Estrogen-Responsive Breast Cancer Cells.Recent Pat Anticancer Drug Discov. 2020;15(4):329-340. doi: 10.2174/1574892815666200929145236. Recent Pat Anticancer Drug Discov. 2020. PMID: 32990541
-
History and Function of the Lactate Receptor GPR81/HCAR1 in the Brain: A Putative Therapeutic Target for the Treatment of Cerebral Ischemia.Neuroscience. 2023 Aug 21;526:144-163. doi: 10.1016/j.neuroscience.2023.06.022. Epub 2023 Jun 29. Neuroscience. 2023. PMID: 37391123 Review.
-
Lactate/GPR81 signaling and proton motive force in cancer: Role in angiogenesis, immune escape, nutrition, and Warburg phenomenon.Pharmacol Ther. 2020 Feb;206:107451. doi: 10.1016/j.pharmthera.2019.107451. Epub 2019 Dec 10. Pharmacol Ther. 2020. PMID: 31836453 Review.
Cited by
-
GPR81 nuclear transportation is critical for cancer growth and progression in lung and other solid cancers.World J Clin Oncol. 2025 Aug 24;16(8):107208. doi: 10.5306/wjco.v16.i8.107208. World J Clin Oncol. 2025. PMID: 40901330 Free PMC article.
-
Pan-Cancer Insights: A Study of Microbial Metabolite Receptors in Malignancy Dynamics.Cancers (Basel). 2024 Dec 15;16(24):4178. doi: 10.3390/cancers16244178. Cancers (Basel). 2024. PMID: 39766077 Free PMC article.
-
The lactylation modification of proteins plays a critical role in tumor progression.Front Oncol. 2025 Mar 19;15:1530567. doi: 10.3389/fonc.2025.1530567. eCollection 2025. Front Oncol. 2025. PMID: 40190564 Free PMC article. Review.
-
Lactate and lactylation in breast cancer: current understanding and therapeutic opportunities.Cancer Biol Med. 2025 Jul 16;22(7):789-805. doi: 10.20892/j.issn.2095-3941.2025.0173. Cancer Biol Med. 2025. PMID: 40671416 Free PMC article. Review.
-
Metabolic Signature of Warburg Effect in Cancer: An Effective and Obligatory Interplay between Nutrient Transporters and Catabolic/Anabolic Pathways to Promote Tumor Growth.Cancers (Basel). 2024 Jan 24;16(3):504. doi: 10.3390/cancers16030504. Cancers (Basel). 2024. PMID: 38339256 Free PMC article. Review.
References
-
- Elingaard-Larsen LOR, M.G.; Sorensen, E. E.; Pedersen, S. F. : How reciprocal interactions between the tumor microenvironment and ion transport proteins drive cancer progression. Reviews of Physiology, Biochemistry, and Pharmacology 2020, In press. - PubMed
-
- DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, Thompson CB. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci U S A. 2007;104(49):19345–19350. doi: 10.1073/pnas.0709747104. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

