Polystyrene nanoplastic exposure induces excessive mitophagy by activating AMPK/ULK1 pathway in differentiated SH-SY5Y cells and dopaminergic neurons in vivo
- PMID: 37993864
- PMCID: PMC10664492
- DOI: 10.1186/s12989-023-00556-4
Polystyrene nanoplastic exposure induces excessive mitophagy by activating AMPK/ULK1 pathway in differentiated SH-SY5Y cells and dopaminergic neurons in vivo
Abstract
Background: Microplastics and nanoplastics (MNPs) are emerging environmental contaminants detected in human samples, and have raised concerns regarding their potential risks to human health, particularly neurotoxicity. This study aimed to investigate the deleterious effects of polystyrene nanoplastics (PS-NPs, 50 nm) and understand their mechanisms in inducing Parkinson's disease (PD)-like neurodegeneration, along with exploring preventive strategies.
Methods: Following exposure to PS-NPs (0.5-500 μg/mL), we assessed cytotoxicity, mitochondrial integrity, ATP levels, and mitochondrial respiration in dopaminergic-differentiated SH-SY5Y cells. Molecular docking and dynamic simulations explored PS-NPs' interactions with mitochondrial complexes. We further probed mitophagy's pivotal role in PS-NP-induced mitochondrial damage and examined melatonin's ameliorative potential in vitro. We validated melatonin's intervention (intraperitoneal, 10 mg/kg/d) in C57BL/6 J mice exposed to 250 mg/kg/d of PS-NPs for 28 days.
Results: In our in vitro experiments, we observed PS-NP accumulation in cells, including mitochondria, leading to cell toxicity and reduced viability. Notably, antioxidant treatment failed to fully rescue viability, suggesting reactive oxygen species (ROS)-independent cytotoxicity. PS-NPs caused significant mitochondrial damage, characterized by altered morphology, reduced mitochondrial membrane potential, and decreased ATP production. Subsequent investigations pointed to PS-NP-induced disruption of mitochondrial respiration, potentially through interference with complex I (CI), a concept supported by molecular docking studies highlighting the influence of PS-NPs on CI. Rescue experiments using an AMPK pathway inhibitor (compound C) and an autophagy inhibitor (3-methyladenine) revealed that excessive mitophagy was induced through AMPK/ULK1 pathway activation, worsening mitochondrial damage and subsequent cell death in differentiated SH-SY5Y cells. Notably, we identified melatonin as a potential protective agent, capable of alleviating PS-NP-induced mitochondrial dysfunction. Lastly, our in vivo experiments demonstrated that melatonin could mitigate dopaminergic neuron loss and motor impairments by restoring mitophagy regulation in mice.
Conclusions: Our study demonstrated that PS-NPs disrupt mitochondrial function by affecting CI, leading to excessive mitophagy through the AMPK/ULK1 pathway, causing dopaminergic neuron death. Melatonin can counteract PS-NP-induced mitochondrial dysfunction and motor impairments by regulating mitochondrial autophagy. These findings offer novel insights into the MNP-induced PD-like neurodegenerative mechanisms, and highlight melatonin's protective potential in mitigating the MNP's environmental risk.
Keywords: Complex I; Melatonin; Microplastics and nanoplastics; Mitochondrial dysfunction; Mitophagy; Neurotoxicity.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


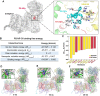


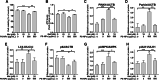


References
-
- Chen H, Chen YH, Xu YB, Xiao CQ, Liu JC, Wu RR, et al. Different functional areas and human activities significantly affect the occurrence and characteristics of microplastics in soils of the Xi'an metropolitan area. Sci Total Environ. 2022;852:158581. doi: 10.1016/j.scitotenv.2022.158581. - DOI - PubMed
-
- Wen SY, Zhao Y, Wang MQ, Yuan HB, Xu HY. Micro(nano)plastics in food system: potential health impacts on human intestinal system. Crit Rev Food Sci. 2022:1–19. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 2022A1515111098/Guangdong-Dongguan Joint grant
- 2022M721486/Chinese Postdoctoral Science Foundation
- 82304177/National Natural Science Foundation of China
- 22106025/National Natural Science Foundation of China
- 82273656/National Natural Science Foundation of China
- 2022A0505050035/Science and Technology Project of Guangdong Province, China
- 2022A1515010610/Guangdong Basic and Applied Basic Research Foundation
- 2017B030314035/Guangdong Provincial Key Laboratory of Tropical Disease Research
- 202212121034/National Training Program of Innovation and Entrepreneurship for Undergraduates
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

