Circular RNAs in renal cell carcinoma: from mechanistic to clinical perspective
- PMID: 37993909
- PMCID: PMC10664289
- DOI: 10.1186/s12935-023-03128-w
Circular RNAs in renal cell carcinoma: from mechanistic to clinical perspective
Erratum in
-
Correction: circular RNAs in renal cell carcinoma: from mechanistic to clinical perspective.Cancer Cell Int. 2024 Jan 18;24(1):40. doi: 10.1186/s12935-023-03184-2. Cancer Cell Int. 2024. PMID: 38238742 Free PMC article. No abstract available.
Abstract
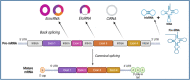
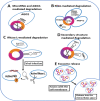
CircRNAs, a special type of noncoding RNAs characterized by their stable structure and unique abilities to form backsplicing loops, have recently attracted the interest of scientists. These RNAs are abundant throughout the body and play important roles such as microRNA sponges, templates for transcription, and regulation of protein translation and RNA-binding proteins. Renal cancer development is highly correlated with abnormal circRNA expression in vivo. CircRNAs are currently considered promising targets for novel therapeutic approaches as well as possible biomarkers for prognosis and diagnosis of various malignancies. Despite our growing understanding of circRNA, numerous questions remain unanswered. Here, we address the characteristics of circRNAs and their function, focusing in particular on their impact on drug resistance, metabolic processes, metastasis, cell growth, and programmed cell death in renal cancer. In addition, the application of circRNAs as prognostic and diagnostic biomarkers will be discussed.
Keywords: Biomarker; CircRNA; Renal cell carcinoma; Targeted therapy.
© 2023. The Author(s).
Conflict of interest statement
The authors have no potential conflicts of interest to declare.
Figures

References
-
- Elbe N, Sauter G, Epstein J. Pathology and genetics of tumours of the urinary system and male genital organs: international agency for research on cancer (IARC) Oxford: Oxford University Press; 2004.
-
- Rini BI, Powles T, Atkins MB, Escudier B, McDermott DF, Suarez C, et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet. 2019;393(10189):2404–2415. doi: 10.1016/S0140-6736(19)30723-8. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

